As filed with the Securities and Exchange Commission on November 1, 2021.
Registration Statement No. 333-259358
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
__________________________
Amendment No. 3
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
__________________________
HEARTBEAM, INC.
(Exact name of Registrant as specified in its charter)
__________________________
|
Delaware |
541714 |
47-4881450 |
||||||
|
(State or other jurisdiction of |
(Primary Standard Industrial |
(I.R.S. Employer |
__________________________
Chief Executive Officer
2118 Walsh Avenue, Suite 210
Santa Clara, CA 95050
Telephone: 408-899-4443
(Address and telephone number of principal executive offices)
__________________________
Corporation Trust Center
1209 Orange Street
Wilmington New Castle County, De 19801
Telephone: (866) 539-8692
(Name, address and telephone number of agent for service)
__________________________
Copies to:
|
Joseph M. Lucosky, Esq. |
Ralph V. De Martino, Esq. Cavas Pavri, Esq. Schiff Hardin LLP 901 K Street NW Suite 700 Washington, DC 20001 Telephone: (202) 778-6400 Facsimile: (202) 778-6460 |
__________________________
Approximate Date of Commencement of Proposed Sale to the Public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|||
|
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|||
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act ☐
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered |
Proposed |
Amount of |
|||||
|
Units, each consisting of one Common Stock, par value $.0001 per share, and one Warrant to purchase Common Stock(2) |
$ |
20,556,250 |
$ |
1,905.56 |
|
||
|
Common Stock included as part of the Units(3) |
|
|
(4 |
) |
|||
|
Warrants to purchase Common Stock included as part of the Units(3) |
|
|
(4 |
) |
|||
|
Common Stock issuable upon exercise of the Warrants(3) |
$ |
20,556,250 |
$ |
1,905.56 |
|
||
|
Representative’s warrant(3) |
|
|
|
|
|
||
|
Common stock underlying Representative’s warrant |
$ |
1,438,938 |
|
|
|
||
|
Total |
$ |
42,551,438 |
$ |
3,811.12 |
|
||
____________
(1) Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price of the securities registered hereunder to be sold by the registrant.
(2) Includes Units consisting of Common Stock and Warrants which may be issued on exercise of a 30-day option granted to the Underwriters to cover over-allotments, if any.
(3) Pursuant to Rule 416, the securities being registered hereunder include such indeterminate number of additional securities as may be issuable to prevent dilution resulting from stock splits, stock dividends or similar transactions.
(4) No separate registration fee required pursuant to Rule 457(g) under the Securities Act.
(5) Filing fee has been previously paid.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state or jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED NOVEMBER 1, 2021
PRELIMINARY PROSPECTUS

HeartBeam, Inc.
2,750,000 Units
This is an initial public offering of units of securities (the “Units) of HeartBeam, Inc (the “Company” or “HeartBeam”). Each Unit consists of (a) one share of our Common Stock (“Common Stock”) and (b) Warrant (the “Warrants”) to purchase one share of our Common Stock at an exercise price equal to $ % of initial public offering price per Unit. The shares of our Common Stock and the Warrants are immediately separable and will be issued separately but will be purchased together in this offering. We anticipate that the initial public offering price per Unit will be between $5.50 and $6.50 per Unit.
We will not issue fractional shares in connection with the exercise of Warrants. Each warrant will become exercisable upon completion of this offering and will expire five (5) years from the date of issuance.
We have granted The Benchmark Company LLC (“Benchmark”), the lead underwriter of this offering, a 30-day option to purchase up to an additional 412,500 shares of Common Stock and/or Warrants to purchase Common Stock to cover over-allotments, if any.
Prior to this offering, there has been no public market for our Common Stock or Warrants. We have applied to have our Common Stock and Warrants listed on the NASDAQ Capital Market, or NASDAQ, under the symbol “BEAT” and “BEATW”, respectively. The Common Stock and Warrants comprising the Units will begin separate trading immediately upon issuance of the Units.
Investing in our securities is highly speculative and involves a high degree of risk. You should carefully consider the risks and uncertainties described under the heading “Risk Factors” beginning on page 13 of this prospectus before making a decision to purchase our securities.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
|
Per Unit |
Total |
|||||
|
Public offering price(1) |
$ |
$ |
||||
|
Underwriting discounts and commissions |
$ |
$ |
||||
|
Proceeds to HeartBeam, Inc. before expenses |
$ |
$ |
||||
____________
(1) Consists of $ attributable to the Common Stock and $0.01 attributable to the Warrant included in the Unit.
The Company has granted a 30-day option to the representative of the underwriters to purchase up to an additional 412,500 shares of Common Stock and/or Warrants to purchase Common Stock to cover over-allotments, if any.
For a description of the other compensation to be received by the underwriters, please see “Underwriting” beginning on page 77.
The underwriters expect to deliver the Units to purchasers in the offering on or about , 2021.
Book-Running Manager
THE BENCHMARK COMPANY
The date of this prospectus is , 2021.
ABOUT THIS PROSPECTUS
In this prospectus, unless the context suggests otherwise, references to “the Company,” “HeartBeam”, “we,” “us,” and “our” refer to HeartBeam, Inc.
This prospectus describes the specific details regarding this offering, the terms and conditions of the Units being offered hereby and the risks of investing in the Company’s Units. You should read this prospectus and the additional information about the Company described in the section entitled “Where You Can Find More Information” before making your investment decision.
Neither the Company, nor any of its officers, directors, agents, representatives or underwriters, make any representation to you about the legality of an investment in the Company’s Units. You should not interpret the contents of this prospectus to be legal, business, investment or tax advice. You should consult with your own advisors for that type of advice and consult with them about the legal, tax, business, financial and other issues that you should consider before investing in the Company’s securities.
ADDITIONAL INFORMATION
You should rely only on the information contained in this prospectus and in any accompanying prospectus supplement. No one has been authorized to provide you with different or additional information. The shares of Units are not being offered in any jurisdiction where the offer is not permitted. You should not assume that the information in this prospectus or any prospectus supplement is accurate as of any date other than the date on the front of such documents.
TRADEMARKS AND TRADE NAMES
This prospectus includes trademarks that are protected under applicable intellectual property laws and are the Company’s property. This prospectus also contains trademarks, service marks, trade names and/or copyrights of other companies, which are the property of its owners. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that the Company will not assert, to the fullest extent under applicable law, its rights or the right of the applicable licensor to these trademarks and trade names.
INDUSTRY AND MARKET DATA
Unless otherwise indicated, information contained in this prospectus concerning the Company’s industry and the markets in which it operates, including market position and market opportunity, is based on information from management’s estimates, as well as from industry publications and research, surveys and studies conducted by third parties. The third-party sources from which the Company has obtained information generally state that the information contained therein has been obtained from sources believed to be reliable, but the Company cannot assure you that this information is accurate or complete. The Company has not independently verified any of the data from third-party sources nor has it verified the underlying economic assumptions relied upon by those third parties. Similarly, internal company surveys, industry forecasts and market research, which the Company believes to be reliable, based upon management’s knowledge of the industry, have not been verified by any independent sources. The Company’s internal surveys are based on data it has collected over the past several years, which it believes to be reliable. Management estimates are derived from publicly available information, its knowledge of the industry, and assumptions based on such information and knowledge, which management believes to be reasonable and appropriate. However, assumptions and estimates of the Company’s future performance, and the future performance of its industry, are subject to numerous known and unknown risks and uncertainties, including those described under the heading “Risk Factors” in this prospectus and those described elsewhere in this prospectus, and the other documents the Company files with the Securities and Exchange Commission, or SEC, from time to time. These and other important factors could result in its estimates and assumptions being materially different from future results. You should read the information contained in this prospectus completely and with the understanding that future results may be materially different and worse from what the Company expects. See the information included under the heading “Forward-Looking Statements.”
i
The following summary highlights information contained elsewhere in this prospectus. This summary may not contain all of the information that may be important to you. You should read this entire prospectus carefully, including the sections entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Company’s historical financial statements and related notes included elsewhere in this prospectus. In this prospectus, unless otherwise noted, the terms “the Company,” “HeartBeam” “we,” “us,” and “our” refer to HeartBeam, Inc.
Overview
Company Overview
We are a medical technology company primarily focusing on telemedicine solutions that enable the detection and monitoring of cardiac disease outside a healthcare facility setting. Our aim is to deliver innovative, remote diagnostic and monitoring technologies that can be used for patients anywhere, with initial offerings for ambulatory and emergency room use. Our products require FDA clearance and have not been cleared for marketing (hereinafter “Product” or “Products”.) We believe our Products and services will benefit many stakeholders, including patients, healthcare providers, and healthcare payors. Our initial focus is providing diagnostic data to help physicians with care management of patients with cardiovascular disease. There are two major markets for our initial Products: remote patient monitoring and the hospital Emergency Room (ER). First, we are developing our telehealth Product to address the rapidly growing field of remote patient monitoring. Our telehealth Product is comprised of a credit card sized ECG machine and a powerful cloud-based diagnostic software expert system. We believe that we are uniquely positioned to play a central role in remote monitoring of high-risk coronary artery disease patients, because the studies performed so far have shown that our ischemia detection system is highly accurate. Our powerful ischemia detection system is unlike other ambulatory cardiac monitors currently on the market which focus on arrhythmia detection. Secondly, we are applying our platform technology to create a software tool for detecting heart attacks in the ER environment. This software tool is designed to enable emergency physicians to more accurately and quickly diagnose heart attacks than currently available. Market release of this Product will precede that of the telehealth Product.
To date, we have developed working prototypes for both our telehealth Product and our ER Product. The ER Product is currently undergoing additional engineering work that we believe will make it ready for FDA 510(k) clearance submission. Both Products have been validated in three medical studies, which were designed by Harvard Medical School faculty. Peer reviewed publications that describe the studies and results are in preparation. One peer reviewed medical publication submission is planned for November 2021 and another for January 2022. These two publications will describe results of our two key studies: HIDES and B Score. In early 2022 we plan to publish results of a study on performance of our ER Product in the real-life environment of an ER. In July 2021 we submitted a technology abstract for presentation at the IEEE EMBS 2021 Conference; it was accepted. The abstract describes the technology foundation of our ER Product
The custom software and hardware of our Products, we believe, are classified as Class II medical devices by the FDA. Class II medical devices are those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish special controls. Special controls can include performance standards, post-market surveillance, patient histories and FDA guidance documents. Premarket review and clearance by the FDA for these devices is generally accomplished through the 510(k) or 510(k) de-novo premarket notification process.
HeartBeam has three issued U.S. patents, seven pending U.S. patent applications (two pending provisional applications and five are utility applications). Two of the pending utility applications have been published, all of the remaining five pending cases are unpublished.
Market Overview
Chronic diseases are the number one burden on the healthcare system, driving up costs each year, and cardiovascular illnesses are one of the top contributors. Regulators, payors and providers are focused on shifting the diagnosis and management of these conditions to drive better outcomes at lower cost. Connected medical solutions are expanding rapidly and are projected to reach $155 billion by 2026, a compound annual growth rate (CAGR) of 17%. These solutions are socio-technical models for healthcare management and delivery using technology to provide healthcare services remotely and aim to maximize healthcare resources and provide increased, flexible opportunities for consumers to engage with clinicians and better self-manage their care, using readily available consumer technologies
1
to deliver patient care outside of the hospital or doctor’s office. The types of companies that make up this market include Accenture, IBM, SAP, GE Healthcare, Oracle, Microsoft, Airstrip Technology, Medtronic, Allscripts, Boston Scientific, Athenahealth, Cerner, Philips, Agamatrix, Qualcomm, and AliveCor.
The market for remote patient monitoring (RPM), is projected to reach $31.3 billion by 2023. In 2019, 1,800 hospitals in the US were using mobile applications to improve risk management and quality of care. The number in 2020 was likely larger as the onset of the COVID-19 pandemic greatly accelerated use and acceptance of telehealth by both patients and healthcare providers.
Cardiovascular disease is the number one cost to the healthcare system and is estimated to be responsible for 1 in every 6 healthcare dollars spent in the US. As cardiovascular disease is the leading cause of death worldwide, early detection, diagnosis, and management of chronic cardiac conditions are necessary to relieve the increasing burden on the healthcare infrastructure. Diagnostic tests such as Electrocardiograms (ECGs) are used to detect, diagnose and track numerous cardiovascular conditions. With advances in mobile communications, diagnostic monitoring of cardiac conditions is increasingly occurring outside the hospital.
Our initial telemedicine technology Product will address the heart attack detection market as well as the market to monitor coronary artery disease (CAD) patients who are typically at high risk for a heart attack. Currently there are no products on the market that are user friendly, easy to carry, and always with the patient in order to provide physicians and patients with timely and highly accurate information about potential Acute Coronary Syndrome (ACS) and Myocardial Infarction (MI) events. A tool that is always with the patient, that decreases time to intervention, and that decreases the number of unnecessary ER visits by chest pain patients would have a significant effect on saving lives and healthcare dollars. We believe our technology will address this problem and will provide a convenient, cost-effective, integrated telehealth solution, including software and hardware for physicians and their patients. There are approximately 18 million people in the US who are considered at high risk for a heart attack, including 8 million who already have had prior intervention for MI’s and are therefore considered to be at extreme risk.
In the US, mobile cardiac tests are primarily conducted through outsourced Independent Diagnostic Testing Facilities (IDTFs) or as part of a Remote Patient Monitoring (RPM) system. Reimbursement rates vary depending on the use case and generally are based on the value a technology offers to patients and healthcare providers. Actual reimbursed pricing is set by the Centers for Medicare & Medicaid Services (CMS). Reimbursement rates for private insurers typically provide for similar or better reimbursement rates when compared to those set by the Government for Medicare and Medicaid.
In the ER environment, early and accurate diagnosis of a chest pain patient who is potentially having a heart attack is of immense importance. Guidelines state that every chest pain patient in an ER must receive an ECG within 10 minutes of presentation. The accuracy of these initial ECGs is only approximately 75%. The need for increased ECG accuracy in detecting a heart attack in the ER is well defined, and an improved solution could result in saved lives and healthcare dollars. We are currently developing a version of our ER Product that will meet all requirements for FDA 510(k) clearance submission. We believe this Product will offer a marked increase in the accuracy of heart attack detection in ERs. There are approximately 5,000 ER departments in the US.
Products and Technology
The foundation of our novel technology is the concept of vectorcardiography (VCG), a technology that has long been seen as superior to ECGs in detecting MIs, but is no longer used clinically because of the difficulty experienced by physicians interpreting the output. We solved the crucial problem of recording three orthogonal (x, y and z) projections of the heart vector with a device that is sized like a credit card. The thickness of our credit card sized ECG signal collection device is about 1/8 inch (3 mm) and it weighs about 1 ounce (28 grams). The core technology consists of a series of patented inventions and associated algorithms. In addition to using VCG to get a more complete 3D characterization of cardiac activity, we use the concept of a baseline. Our MI marker is a differential marker that measures the change in cardiac parameters between an asymptomatic (baseline) recording and the symptomatic recording. It is personalized for every patient as every patient has a unique baseline. Our increase in diagnostic performance in detecting MIs, when compared to a panel of cardiologists, is attributed to a richer cardiac information set offered by VCG and the fact that our MI marker compares the baseline and symptomatic recordings and does that in the 3D space of VCG.
2
This novel technology has resulted in two key Products to date: a telehealth Product for high-risk cardiovascular patients and a powerful cloud-based diagnostic expert and MI detection system for ERs.
Our telehealth ECG collection device is the size of a credit card and records cardiac signals with integrated electrodes rather than wires or self-adhesive electrodes. Unlike a standard 12-lead ECG machine that records signals in empirically determined locations on a human body, our approach is focused on recording three projections of the heart vector. The successful recording of the projections of the heart vector enables the synthesis of a 12-lead signal set as well internal algorithmic diagnostic work in the space of 3D heart vectors.
There are obvious ease of use advantages when comparing our handheld device that fits in a wallet and can be instantly self-applied versus the current 12-lead ECG machine that requires a trained professional to apply. In addition, there are diagnostic performance advantages, including, based on our initial study, increased accuracy in diagnosing MIs. The system is used by patients at home or elsewhere, with help from their physicians, to assess whether their chest pain is truly the result of an MI.
Our telehealth system will be a prescription-only mobile health system intended for individuals with known or suspected heart disease, especially coronary artery disease (CAD). It helps guide physicians in choosing the best course of action for their patients who experience chest pain outside of a medical facility. Our system will bring a medical grade ECG to patients and will enable them to receive a plan of action from a physician in a timely manner. At the time of onboarding and at regularly scheduled time intervals, patients record a baseline 30 second cardiac reading using our device. When a patient experiences symptoms, such as irregular heartbeats or chest pain, the patient can simply open the smartphone app and press the credit card sized device against the chest to collect signals that can be converted to a 12-lead ECG. This derived 12-lead ECG is sent to the physician overlayed with the patient’s derived baseline ECG recording. In addition, the patient provides input on their symptoms that are sent, along with the ECG data, to the cloud for interpretation by a physician. A cloud-based algorithm processes the signals and displays the symptom description and patient history to a physician to analyze and prescribe an action plan. From start to finish, the process takes just a few minutes.
The telehealth system consists of:
1. A credit card sized cardiac electrical signal collection device. The device captures cardiac signals that represent x, y and z projections of the heart vector and transmits them via Bluetooth connection to a smartphone. It is always with the patient as it easily fits in a wallet. It is easy to use as all that is required of the patient is that the device be pressed against the chest.
2. A smartphone application that receives the cardiac signals from the HeartBeam signal collection device. The app has several functions: guiding the patient through the signal collection, asking about symptoms, displaying the status of the data collection, and notifying the patient of the plan of action as determined by a physician. In addition, the app will contain HIPAA-compliant video conferencing or text capabilities for the healthcare provider to communicate directly with the patient.
3. A cloud-based software system that serves three basic functions: (1) Performing a final check of the ECG signal quality, (2) Synthesizing a 12-lead ECG from the measured (recorded) 3 vector leads, and (3) Preparing a summary report for the physician. In order to facilitate a more accurate physician interpretation of the data, the software overlays the patient’s synthesized baseline 12 lead ECG waveform on the synthesized 12 lead ECG waveform from the current event. To ensure high signal quality, the system checks for noise levels in the recorded signals. Those signals that can be effectively filtered are accepted and those that have a noise level above an empirically established threshold are rejected. If a recorded signal is rejected, the user is asked to repeat the recording.
4. A web-based physician portal, which displays all of the relevant information for the physician to analyze: patient history, symptoms, baseline and current readings, synthesized 12 lead ECG, and recorded 3 vector leads. The HeartBeam physician portal assists physicians with their diagnostic interpretation by providing both the baseline 12-lead synthesized ECG and the 12-lead synthesized ECG that is under evaluation.
The market release of our telehealth Product will be in two generations. The generation 1 product will have a limited feature set and will offer to the physician a pair of baseline and symptomatic ECGs and a symptoms report. This product is an excellent match for existing CPT remote patient monitoring reimbursement codes. The generation 2 product will
3
feature our proprietary MI marker as well as our diagnostic suggestion in addition to all features of Generation 1 product. Since Generation 2 will offer much increased medical value, we will seek a unique reimbursement code for this product.
The same core technology is used in the ER Product. In this application, the increased accuracy of detecting MIs by the ECG is of utmost importance. An ECG is the first diagnostic test a chest pain patient receives in the ER and it has a major impact on the patient’s subsequent clinical path. The ER Product has no hardware and introduces minimal change to the standard of care chest pain diagnostic path. It uses a baseline standard 12-lead ECG from the patient’s Electronic Medical Record (EMR) and the chest pain ECG that is being evaluated. It converts both of them to a VCG representation and utilizes our proprietary 3D VCG differential marker. An initial clinical study indicates that the ER software Product offers considerable improvement in the accuracy of MI detection compared to a panel of experienced cardiologists interpreting a standard 12 lead ECG. The importance of increased accuracy of the first ECG interpretation for a chest pain patient presenting to an ER is significant, potentially leading to faster intervention or avoiding unnecessary activation of the cardiac catheterization lab.
Market Opportunity
ECGs are key diagnostic tests utilized in the diagnosis and monitoring of cardiovascular disease, the number one cause of death worldwide. In the US in 2016, there were 121.5 million adults living with cardiovascular disease and 18.3 million adults with diagnosed coronary artery disease. The market size is increasing, due to an aging population and lifestyle choices.
Every 40 seconds someone in the US has a heart attack, or myocardial infarction (MI). Unfortunately, there is no way for patients to tell whether the symptoms they are experiencing are due to an MI, or some other more benign condition such as indigestion. As a result, patients often ignore symptoms and delay seeking care, which leads to worse outcomes and increased mortality. On the other hand, many patients who go to the Emergency Room with chest pain are not experiencing an MI. Chest pain is the second most common reason for an ER visit, yet fewer than 20% of chest pain ER visits result in a diagnosis of a life-threatening condition. These unnecessary ER visits lead to well over $10 billion in unnecessary healthcare expenditures.
Most ECGs are conducted in a healthcare facility setting using a 12-lead ECG machine, the gold standard. ECGs taken outside of healthcare facilities are expected to grow more quickly than in-hospital ECGs. Monitoring cardiac patients outside of a hospital is a fast growing trend, as it is less expensive and provides a better patient experience. However, while ambulatory cardiac monitoring devices are often much easier for patients to use, they have fewer leads than the gold standard and therefore cannot offer as comprehensive a picture of cardiac health.
While a standard 12-lead ECG readout is of great medical value, it is simply impractical to have a machine next to patients when they experience symptoms outside the clinical setting, since recording the event requires attaching multiple electrodes to the patient’s body with professional assistance. While existing technologies use predominantly single lead ECG devices to monitor arrhythmias, these technologies do not provide information to the physician on the presence of life threatening conditions of ACS or heart attacks.
We believe our telehealth technology addresses these market needs and has several key attributes that make it a good fit for these patients. Our telehealth Product is generally used when symptoms occur and offers the potential for lifelong patient usage. The device is always near the patient and ready to be used for recording a cardiac event. It enables real-time cardiac data transmission during a telemedicine visit. It offers a recorded 3 vector lead set of signals and a 12-lead derived ECG set of signals. Physicians will typically prescribe our solution to chronic cardiovascular patients for long term monitoring, thereby enabling prolonged data collection and delivering a more complete picture for diagnosis. This will also enable the use of artificial intelligence on our future database that will have a unique set of longitudinal 12-lead ECGs for patients.
Market Strategy
Our goal is to establish our products as key solutions for cardiology practices and hospital ERs. Our efforts to enter the market involve establishing clinical evidence and demonstrating the cost-effectiveness of adopting our products. For both the telehealth and the ER Products, the initial geographic market is the United States.
4
We believe that both the telehealth and ER Products will be subject to the US FDA’s 510(k) review process. We are in the process of preparing regulatory submissions for our ER Product and Generation 1 telehealth Product.
For the telehealth Product, the primary customers are cardiology practices and the cardiology departments of hospitals. Healthcare insurers are another important customer, as they will benefit from the reduced costs to the healthcare system. We are working to develop new clinical studies and publish results of completed clinical studies and plan to demonstrate real world cost-effectiveness of the use of the solution.
A key element of our strategy is obtaining reimbursement for the telehealth Product. This strategy has two stages in full alignment with our Generation 1 and Generation 2 telehealth product introduction plans. In the short term, we expect that physicians will use existing Remote Patient Monitoring (RPM) reimbursement codes. We believe that our Generation 1 telehealth Product will be a compelling offering among RPM technologies, as it will be uniquely positioned to assess ACS and heart attacks among high-risk cardiac patients. In the longer term, we will conduct additional clinical trials that demonstrate the clinical efficacy and cost effectiveness of our next generation, full featured Generation 2 Product and will work to secure a new CPT code and reimbursement specific to Generation 2 telehealth solution.
RPM codes pay practices for providing covered services. The main RPM codes relevant for our Generation 1 telehealth Product are:
• CPT 99453: Remote monitoring of physiologic parameter(s), initial; set-up and patient education on use of equipment
• CPT 99454: Remote monitoring of physiologic parameter(s), initial; device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 days
• CPT 99457: Remote physiologic monitoring treatment management services, clinical staff/physician/ other qualified health care professional time in a calendar month requiring interactive communication with the patient/caregiver during the month; initial 20 minutes
• CPT 99458: Remote physiologic monitoring treatment management services, clinical staff/physician/ other qualified health care professional time in a calendar month requiring interactive communication with the patient/caregiver during the month; additional 20 minutes
CPT 99453 is paid one-time per patient, with the average CMS payment rate of $21. The technical code CPT 99454 and the professional code CPT 99457 are paid monthly, with a combined average CMS payment rate of $119. Private payers may pay at different amounts.
Practices will bill payers for monthly services related to the core HeartBeam telehealth Product, potentially bundled with a third-party blood pressure measurement device. Under this model, the company will negotiate with payers for a per patient per month fee for the ongoing HeartBeam telehealth service, which also will include an amortized charge for the cost of the device.
We will explore other business models. For example, hospitals face CMS penalties if their 30-day readmission rates for patients who are discharged after an MI exceed certain thresholds. These CMS penalties are levied on all hospital CMS payments, so the impact can be significant. Our telehealth Product can be a tool to help hospitals manage these patients after discharge. We will explore models in which hospitals pay for the device and for the initial 30 days of service. In addition, we will explore models for value-based care, in which the use of the telehealth Product reduces overall costs.
We expect to develop a direct sales force for our telehealth Product and to target large hospitals and integrated practices. These are sophisticated customers, and we will use technical presentations, peer reviewed clinical data, and demonstration projects to achieve penetration of this market. We will continue to expand our medical advisory board, will conduct clinical trials with leading cardiologists to increase the body of evidence, and will establish reference sites among these customers.
5
Our long-term strategy is to generate sufficient evidence of clinical efficacy and cost-effectiveness to generate reimbursement coverage and payment specifically for the HeartBeam telehealth Generation 2 solution. We expect to be able to demonstrate significant clinical benefits for patients and savings to the health care system, justifying reimbursement levels well in excess of the amount paid through the RPM pathway.
For the telehealth Product, our primary marketing strategy will focus on the medical community with continued validation of clinical efficacy and cost-effectiveness and the establishment of reference sites. We will also create educational materials and provide other support to help educate our customers’ patients.
For the ER Product, the primary customers are acute care facilities. As with the telehealth Product, we will publish clinical studies on the effectiveness of the Product. In addition, we will develop financial models demonstrating the cost-effectiveness of the approach and will establish reference sites who are using the Product. We do not expect to obtain specific reimbursement for the ER Product but intend to demonstrate that the purchase of the software would result in clinical and economic benefits and potentially reduced malpractice legal exposure for ER provider institutions.
We will establish a direct sales network with relationships and experience selling to ERs. In addition, we will identify leading Emergency Physicians to conduct clinical studies and generate real-world experience with the Product.
Clinical Data
HeartBeam has performed three clinical studies to assess performance of our technologies.
In the first study (HeartBeam Ischemia Detection Study — HIDES), we collected electrical signal data on patients whose coronary arteries were occluded during a Percutaneous Coronary Intervention (PCI), using simultaneously a traditional 12-lead ECG and our vector signal based device. Our VCG-based signal interpretation system had significantly (21%) higher accuracy in detecting ischemia as more fully discussed in the Business Section.
In a second study (B Score), the HeartBeam diagnostic engine, using ECG, symptoms, and history, matched the diagnostic performance of expert cardiologists in detecting the presence of MIs in patients presenting to an ER with chest pain. This result indicates that the quality of the diagnostic advice produced by our expert system will be extremely valuable to the physician who is assessing the condition of a patient in a telehealth environment.
The third study, ISPEC, which assessed the false positive rate for non-symptomatic patients, is relevant in a telehealth situation. It is important that the system have a low false positive rate when patients are conducting baseline recordings, which are required on at least a monthly basis. The study yielded no false positives.
All three studies are being prepared for peer-reviewed publication.
Intellectual Property
Our innovations are protected with a strong patent portfolio. For a limited number of aspects of our proprietary technology, we rely on trade secret protection. It is our view that the combination of these two methods of intellectual property protection maximizes our chances for success.
HeartBeam has three issued U.S. utility patents, five pending U.S. utility patent applications and two pending provisional applications.
Research and Development
The primary objective of our research and development program is to provide innovative, user friendly solutions with high medical value. To date, we have been highly successful in developing our initial products. The emphasis has been on developing a user-friendly solution that is always with the patient and that provides assistance to physicians in diagnosing heart attacks in chest pain patients.
Our Research and Development team is largely based in Belgrade, Serbia. We have assembled a highly capable team currently consisting of seven PhD level contributors who are credited with developing our key inventions and patents. The diverse group includes two nuclear physicists, three signal processing specialists, and two biomedical engineers.
6
We plan to utilize this team in the future and expect that the key members of this team will transition to full time employees. Future research and development efforts will focus on the application of signal processing and artificial intelligence to address a range of cardiac conditions.
Future Products
Our core technology — the heart vector approach adopted and invented by our scientific team — is a platform technology that can provide diagnostic solutions to a variety of cardiovascular patients. Our plans call for expanding solutions that diagnose all major cardiac conditions that are diagnosed by ECGs.
Our future plans include the development of a 12-lead capable patch ECG monitor that will provide advantages over existing single-lead ECG patch products such as the Zio-XT from iRhythm Technologies, Inc. Our approach will offer a 12-lead ECG with a patch that is very similar, in dimensions and look and feel, to the currently available single lead ECG patches. We believe providing standard of care 12-lead ECG capabilities will have significant diagnostic advantages over a single lead patch.
While our initial telehealth Product is powered by a software expert system that serves as a diagnostic aid to a physician, we will develop an AI based diagnostic system that will supplement our diagnostic expert system.
Summary of Risk Factors
Our business and our ability to execute our business strategy are subject to a number of risks of which you should be aware of before you decide to buy our Units. In particular, you should carefully consider following risks, which are discussed more fully in “Risk Factors” beginning on page 13 of this prospectus:
• We have a limited operating history upon which investors can evaluate our future prospects.
• Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern.
• We have no revenues and we cannot predict when we will achieve first revenues and be sustained.
• We may never complete the development and commercialization of products that we are currently developing and future development of new generations of any of our other proposed products.
• We may not meet our product development and commercialization milestones.
• Our business is dependent upon physicians utilizing and prescribing our solution; if we fail to engage physicians to utilize our solution, our revenues may never materialize or may not meet our projections.
• We are subject to extensive governmental regulations relating to the manufacturing, labeling and marketing of our products.
• If we are not able to both obtain and maintain adequate levels of third-party reimbursement for our products, it would have a material adverse effect on our business.
• Changes in reimbursement practices of third-party payers could affect the demand for our products and services and our revenue levels.
• We may experience difficulty in obtaining reimbursement for our services from commercial payors that consider our technology to be experimental and investigational, which would adversely affect our revenue and operating results.
• Reimbursement by Medicare is highly regulated and subject to change; our failure to comply with applicable regulations, could decrease our expected revenue and may subject us to penalties or have an adverse impact on our business.
• Consolidation of commercial payors could result in payors eliminating coverage of mobile cardiac monitoring solutions or reducing reimbursement rates.
• Product defects could adversely affect the results of our operations.
7
• Interruptions or delays in telecommunications systems or in the data services provided to us by cellular communication providers or the loss of our wireless or data services could impair the delivery of our cardiac monitoring services.
• Interruptions in computing and data management cloud systems could impair the delivery of our cardiac monitoring services.
• We could be exposed to significant liability claims if we are unable to obtain insurance at acceptable costs and adequate levels or otherwise protect ourselves against potential product liability claims.
• We require additional capital to support our present business plan and our anticipated business growth, and such capital may not be available on acceptable terms, or at all, which would adversely affect our ability to operate.
• We cannot predict our future capital needs and we may not be able to secure additional financing.
• The results of our research and development efforts are uncertain and there can be no assurance of the commercial success of our products.
• If we fail to retain certain of our key personnel and attract and retain additional qualified personnel, we might not be able to pursue our growth strategy.
• We will not be profitable unless we can demonstrate that our products can be manufactured at low prices.
• Our dependence on a limited number of suppliers may prevent us from delivering our devices on a timely basis.
• Natural disasters and other events beyond our control could materially adversely affect us.
• The COVID-19 pandemic may negatively affect our operations.
• The industry in which we operate is highly competitive and subject to rapid technological change. If our competitors are better able to develop and market products that are safer, more effective, less costly, easier to use, or are otherwise more attractive, we may be unable to compete effectively with other companies.
• We face competition from other medical device companies that focus on similar markets.
• Unsuccessful clinical trials or procedures relating to products under development could have a material adverse effect on our prospects.
• Intellectual property litigation and infringement claims could cause us to incur significant expenses or prevent us from selling certain of our products.
• If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
• If we are unable to protect our proprietary rights, or if we infringe on the proprietary rights of others, our competitiveness and business prospects may be materially damaged.
• Dependence on our proprietary rights and failing to protect such rights or to be successful in litigation related to such rights may result in our payment of significant monetary damages or impact offerings in our product portfolios.
• Enforcement of federal and state laws regarding privacy and security of patient information may adversely affect our business, financial condition or operations.
• We may become subject, directly or indirectly, to federal and state health care fraud and abuse laws and regulations and if we are unable to fully comply with such laws, the Company could face substantial penalties.
• We may be subject to federal and state false claims laws which impose substantial penalties.
• The price of our Common Stock and Warrants may be subject to wide fluctuations.
8
• The offering price of the Units may not be indicative of the value of our assets or the price at which shares can be resold. The offering price of the Units may not be an indication of our actual value.
• If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
• We may, in the future, issue additional shares of Common Stock, which would reduce investors’ percent of ownership and dilute our share value
• Our need for future financing may result in the issuance of additional securities which will cause investors to experience dilution.
• If our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
• Liability of directors for breach of duty is limited under Delaware law.
• We do not anticipate paying any cash dividends on our Common Stock in the foreseeable future and, as such, capital appreciation, if any, of our Common Stock will be your sole source of gain for the foreseeable future.
• We are an “emerging growth company,” and any decision on our part to comply with certain reduced disclosure requirements applicable to emerging growth companies could make our Common Stock less attractive to investors.
• As a result of our becoming a public company, we will become subject to additional reporting and corporate governance requirements that will require additional management time, resources and expense.
• We have identified weaknesses in our internal controls, and we cannot provide assurances that these weaknesses will be effectively remediated or that additional material weaknesses will not occur in the future.
• Future sales of a substantial number of our Common Stock by our existing shareholders could cause our stock price to decline.
• Future sales and issuances of our Common Stock or rights to purchase Common Stock, including pursuant to our equity incentive plans and outstanding warrants could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
• The Company will have broad discretion in the use of the net proceeds from this offering and may fail to apply these proceeds effectively.
• There is no assurance that an active and liquid trading market in our Common Stock and Warrants will develop.
• There is no guarantee that we will successfully have our Common Stock or Warrants listed on the Nasdaq Capital Market. Even if our Common Stock and Warrants are accepted for listing on the Nasdaq Capital Market, upon our satisfaction of the exchange’s initial listing criteria, the exchange may subsequently delist our Common Stock and Warrants if we fail to comply with ongoing listing standards.
• You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
Corporate History and Information
HeartBeam was incorporated as a C corporation under the laws of the State of Delaware on June 11, 2015. We do not own any subsidiaries.
9
THE OFFERING
|
Securities offered by us |
Units at $ per Unit, each Unit consisting of: • One share of Common Stock; and • Warrant to purchase Common Stock. The Warrants may only be exercised with an exercise price of $ (which will not be less than % of the public offering price of one Unit) per whole Common Stock. The Warrants are exercisable upon completion of this offering and will expire five (5) years from the date of issuance. The Common Stock and the Warrants comprising the Units are immediately separable upon issuance and will be issued separately in this offering. |
|
|
Shares of Common Stock outstanding |
|
|
|
Shares of Common Stock to be |
|
|
|
Number of Warrants outstanding before this offering |
|
|
|
Number of Warrants outstanding after this offering |
|
|
|
Over-allotment option |
The Company has granted the underwriters an over-allotment option. This option, which is exercisable for up to 30 days after the date of this prospectus, permits the underwriters to purchase a maximum of 412,500 additional shares of Common Stock (15% of the shares sold in this offering) and/or Warrants to purchase Common Stock from us to cover over-allotments, if any. |
|
|
Use of Proceeds |
We estimate that the net proceeds from this offering will be approximately $14,695,000, or approximately $16,997,000 if the underwriters exercise their over-allotment option in full, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. The Company intends to use the net proceeds from this offering general working capital and other corporate purposes. See “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering. |
|
|
Description of Warrant |
The exercise price of the Warrants is $ per share (with an exercise price no less than % of the public offering price of one Unit). Each Warrant is exercisable for one share of our Common Stock, subject to adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our Common Stock as described herein. Each Warrant is exercisable upon completion of this offering and will expire five (5) years from the date of issuance. The terms of the Warrants will be governed by a warrant agreement, dated as of the effective date of this offering, between us and Vstock Transfer, as the Warrant Agent. This prospectus also relates to the offering of the Common Stock issuable upon exercise of the Warrants. For more information regarding the Warrants, you should carefully read the section titled “Description of Securities — Warrants” in this prospectus. |
10
|
Representative’s Warrant |
The registration statement of which this prospectus is a part also registers warrants (the “Representative’s Warrants”) to purchase up to 221,375 shares of Common Stock (7% of the number of Common Stock sold in this offering (including any Common Stock and/or Warrants sold pursuant to the over-allotment option) to be issued the underwriters, as a portion of the underwriting compensation payable in connection with this offering, as well as the Common Stock issuable upon the exercise of the Representative’s Warrants. Please see “Underwriting — Representative’s Warrants” for a description of these warrants. |
|
|
Dividend Policy |
The Company has never declared any cash dividends on its Common Stock. The Company currently intends to use all available funds and any future earnings for use in financing the growth of its business and does not anticipate paying any cash dividends for the foreseeable future. See “Dividend Policy.” |
|
|
Trading Symbol |
We have applied to list our Common Stock and Warrants on the Nasdaq Capital Market upon our satisfaction of the exchange’s initial listing criteria. Upon approval to list our Common Stock and Warrants we anticipate that the Common Stock and Warrants, will be listed on the Nasdaq Capital Market under the symbol “BEAT” and “BEATW”, respectively. No assurance can be given that our application will be approved. |
|
|
Risk Factors |
You should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the “Risk Factors” section beginning on page 13 of this prospectus before deciding whether or not to invest in the Company’s Units. |
|
|
Lock-up |
We and our directors, officers and principal stockholders have agreed with the underwriters not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our Common Stock or securities convertible into Common Stock for a period of six months after the date of this prospectus. See “Underwriting” section on page 77. |
____________
(1) The number of shares of Common Stock outstanding is based on shares of Common Stock issued and outstanding as of September 30, 2021 and excludes the following:
• 792,912 shares of Common Stock issuable upon the exercise of outstanding stock options having a weighted average exercise price of $1.11 per share;
• 422,549 shares of Common Stock issuable upon the exercise of outstanding warrants having a weighted average exercise price of $0.11 per share;
• 1,449,574 shares of Common Stock issuable upon conversion of the convertible debt;
• 584,848 shares of Common Stock reserved for future issuance under the Company’s 2015 Equity Incentive Plan (the “2015 Plan”);
Except as otherwise indicated herein, all information in this prospectus reflects or assumes:
• no exercise of the outstanding options described above;
• no exercise of the underwriters’ option to purchase up to an additional 412,500 shares of Common Stock to cover over-allotments, if any;
• excludes shares of Common Stock underlying the Warrants issued as part of the Units; and
• excludes shares of Common Stock underlying the Warrants to be issued to the underwriters in connection with this offering.
• a 1 for 2.75 reverse stock split of our issued and outstanding shares of common stock, which was effected on September 27, 2021 (all share and per share amounts in this prospectus have been presented on a retrospective basis to reflect the reverse stock split).
11
The following summary financial and operating data set forth below should be read in conjunction with the Company’s financial statements, the notes thereto and the other information contained in this prospectus. The summary statement of operations data for the three and six months ended June 30, 2021 and 2020 and the years ended December 31, 2020 and 2019 have been derived from the Company’s audited financial statements appearing elsewhere in this prospectus. The historical results presented below are not necessarily indicative of financial results to be achieved in future periods. The financial statements have been prepared and presented in accordance with generally accepted accounting principles in the United States. You should read this data together with the information under the captions “Capitalization” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results are not necessarily indicative of our future results or any other period. The summary financial data included in this section are not intended to replace the financial statements and the related notes included elsewhere in this prospectus. Share amounts, per share data, share prices, exercise prices and conversion rates have been retroactively adjusted to reflect the 1-for-2.75 reverse stock split of or common effected on September 27, 2021.
|
For the |
For the |
For the |
||||||||||||||||||||||
|
Statement of operations data: |
2021 |
2020 |
2021 |
2020 |
2020 |
2019 |
||||||||||||||||||
|
(In thousands, except share and per share data) |
||||||||||||||||||||||||
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
General and administrative |
$ |
312 |
|
$ |
133 |
|
$ |
446 |
|
$ |
227 |
|
$ |
655 |
|
$ |
253 |
|
||||||
|
Research and development |
|
25 |
|
|
21 |
|
|
54 |
|
|
28 |
|
|
133 |
|
|
41 |
|
||||||
|
Total Operating Expenses |
|
337 |
|
|
154 |
|
|
500 |
|
|
255 |
|
|
788 |
|
|
294 |
|
||||||
|
Loss From Operations |
|
(337 |
) |
|
(154 |
) |
|
(500 |
) |
|
(255 |
) |
|
(788 |
) |
|
(294 |
) |
||||||
|
Net Loss |
$ |
(945 |
) |
$ |
(229 |
) |
$ |
(1,155 |
) |
$ |
(402 |
) |
$ |
(1,068 |
) |
$ |
(536 |
) |
||||||
|
Net loss per share, basic and diluted |
|
(0.26 |
) |
|
(0.06 |
) |
|
(0.31 |
) |
|
(0.11 |
) |
|
(0.29 |
) |
|
(0.16 |
) |
||||||
|
Weighted average common shares outstanding |
|
3,699,762 |
|
|
3,638,315 |
|
|
3,706,550 |
|
|
3,646,159 |
|
|
3,645,945 |
|
|
3,411,104 |
|
||||||
|
Unaudited pro forma net loss per share: |
|
|
|
|
||||||||
|
Pro-forma net loss(1) |
$ |
(414 |
) |
$ |
(624 |
) |
||||||
|
Pro forma net loss per share, basic and diluted |
|
(0.05 |
) |
|
(0.08 |
) |
||||||
|
Shares used to calculate pro forma net loss per common share, basic and diluted |
|
7,713,321 |
|
|
7,720,109 |
|
|
Balance sheet data: |
As of |
Pro Forma |
||||||
|
(In thousands) |
||||||||
|
Cash |
$ |
465 |
|
$ |
15,160 |
|
||
|
Total assets |
$ |
521 |
|
$ |
15,216 |
|
||
|
Convertible notes, net |
$ |
4,194 |
|
$ |
— |
|
||
|
Total liabilities |
$ |
4,785 |
|
$ |
591 |
|
||
|
Accumulated deficit |
$ |
(5,951 |
) |
$ |
(5,951 |
) |
||
|
Total Stockholders’ (Deficit) Equity |
$ |
(4,264 |
) |
$ |
14,625 |
|
||
____________
(1) On a pro forma basis the net loss decreased by $531,000 due to the exclusion of the convertible note amortization during the three and six months ended June 30, 2021.
(2) The pro forma balance sheet data reflects the items described in footnotes (1) above and gives effect to our receipt of estimated net proceeds $14.7 million from the sale of Common Stock that we are offering at an assumed initial public offering price of $5.34 per share, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. It also assumes that the convertible notes are automatically converted at $4.20/share ($6.00 * 70%).
(3) The pro forma as adjusted data is illustrative only and will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing.
A $1.00 increase (decrease) in the anticipated initial public offering price of $6.00 per share, would increase (decrease) each of cash, total assets and total stockholders’ equity by approximately $2.6 million, assuming the number of Units offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable. In addition, the convertible notes of $5.3 million gross would convert at a price that is $0.70 different than the initial conversion price of $ 4.20 per share for a public offering price of $ 6.00. It would be $ 4.90 (increase of $1.00) to $ 3.50 (decrease of $1.00).
12
Investing in our Units involves a high degree of risk. Prospective investors should carefully consider the risks described below, together with all of the other information included or referred to in this prospectus, before purchasing our Units. There are numerous and varied risks that may prevent the Company from achieving its goals. If any of these risks actually occurs, the Company’s business, financial condition or results of operations may be materially adversely affected. In such case, the trading price of our Common Stock and Warrants could decline and investors could lose all or part of their investment.
Risks Related to Our Business
We have a limited operating history upon which investors can evaluate our future prospects.
We have a limited operating history upon which an evaluation of our business plan or performance and prospects can be made. The business and prospects of the Company must be considered in the light of the potential problems, delays, uncertainties and complications encountered in connection with a newly established business and new industry. The risks include, but are not limited to, the possibility that we will not be able to develop functional and scalable products and services, or that although functional and scalable, our products and services will not be economical to market; that our competitors hold proprietary rights that preclude us from marketing such products; that our competitors market a superior or equivalent product; that we are not able to upgrade and enhance our technologies and products to accommodate new features and expanded service offerings; or the failure to receive necessary regulatory clearances for our products. To successfully introduce and market our products at a profit, we must establish brand name recognition and competitive advantages for our products. There are no assurances that we can successfully address these challenges and if unsuccessful, we and our business, financial condition and operating results could be materially and adversely affected.
The current and future expense levels of our business are based largely on estimates of planned operations and future revenues rather than experience. It is difficult to accurately forecast future revenues because our business is new and our market has not been developed. If our forecasts prove incorrect, the business, operating results and financial condition of the Company may be materially and adversely affected. Moreover, we may be unable to adjust our spending in a timely manner to compensate for any unanticipated reduction in revenues. As a result, any significant reduction in planned or actual revenues may immediately and adversely affect our business, financial condition and operating results.
Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern.
As described in Note 2 of our accompanying audited financial statements, our auditors have issued a going concern opinion on our December 31, 2020 financial statements, expressing substantial doubt that we can continue as an ongoing business for the next twelve months after issuance of their report based on our current development plans and our operating requirements and us having suffered recurring losses from operations and having a net capital deficiency. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty. If we cannot raise the necessary capital to continue as a viable entity, we could experience a material adverse effect on our business and our stockholders may lose some or all of their investment in us.
We can provide no assurances that any additional sources of financing will be available to us on favorable terms, if at all. Our forecast of the period of time through which our current financial resources will be adequate to support our operations and the costs to support our general and administrative, selling and marketing and research and development activities are forward-looking statements and involve risks and uncertainties.
If we do not succeed in raising additional funds on acceptable terms, we may be unable to complete planned clinical trials or obtain 510(k) clearance of our initial products from the FDA and other regulatory authorities. In addition, we could be forced to delay, discontinue or curtail product development, forego sales and marketing efforts, and forego potential attractive business opportunities. Unless we secure additional financing, we will be unable to fund completion of our initial products and pursue 510(k) clearance from the FDA.
13
We will also need to raise additional capital to expand our business to meet our long-term business objectives. We have no revenues and we cannot predict when we will achieve first revenues and sustained profitability.
We have no revenues and cannot definitely predict when we will achieve revenues and profitability. We do not anticipate generating significant revenues until we successfully develop, achieve regulatory clearance, commercialize and sell our proposed products, of which we can give no assurance. We are unable to determine when we will generate significant revenues from the sale of any such products.
We cannot predict when we will achieve profitability, if ever. Our inability to become profitable may force us to curtail or temporarily discontinue our research and development programs and our day-to-day operations. Furthermore, there can be no assurance that profitability, if achieved, can be sustained on an ongoing basis.
We may never complete the development and commercialization of products that we are currently developing and future development of new generations of any of our other proposed products.
We have no assurance of success as to the completion and of the commercial launch of our products or the completion and development of any new generations of products that are currently under development or other proposed or contemplated products, for any of our target markets. We continue to seek to improve our technologies while we are developing them so that they result in commercially viable products. Failure to improve on any of our technologies could delay or prevent their successful development for our target markets.
Developing any technology into a marketable product is a risky, time consuming and expensive process. You should anticipate that we will encounter setbacks, discrepancies requiring time consuming and costly redesigns and changes, and that there is the possibility of outright failure.
We may not meet our product development and commercialization milestones.
We have established milestones, based upon our expectations regarding our technologies, which we use to assess our progress toward developing our products. These milestones relate to technology development and design improvements as well as dates for achieving development goals. If our products exhibit technical defects or are unable to meet cost or performance goals, our commercialization schedule could be delayed and potential purchasers of our initial commercial products may decline to purchase such products or may opt to pursue alternative products.
We may also experience shortages of the components used in our devices. The contract manufacturing operations that we will use could be disrupted by fire, earthquake or other natural disaster, a labor-related disruption, failure in supply or other logistical channels, electrical outages or other reasons. If there were a disruption to manufacturing facilities, we would be unable to manufacture devices until these manufacturing capabilities are restored or alternative manufacturing facilities are engaged.
Generally, we have met our milestone schedules when making technological advances in our product. We can give no assurance that our development and commercialization schedule will continue to be met as we further develop products currently under development or any of our other future products.
Our business is dependent upon physicians utilizing and prescribing our solution; if we fail to engage physicians to utilize our solution, our revenues may never materialize or may not meet our projections.
The success of our cardiac diagnosis and monitoring business is dependent upon physicians prescribing and utilizing our solution. The utilization of our solution by physicians for use in the prescription of cardiac monitoring is directly influenced by a number of factors, including:
• the ability of the physicians with whom we work to obtain sufficient reimbursement and be paid in a timely manner for the professional services they provide in connection with the use of our monitoring solutions;
• establishing ourselves as a cardiac monitoring technology company by publishing peer reviewed publications showing efficacy of our solutions,
• our ability to educate physicians regarding the benefits of our cardiac monitoring solutions over alternative diagnostic monitoring solutions,
• our demonstrating that our proposed products are reliable and supported by us in the field;
14
• supplying and servicing sufficient quantities of products directly or through marketing alliances; and
• pricing our devices and technology service fees in a medical device industry that is becoming increasingly price sensitive.
If we are unable to drive physician utilization, our revenues may never materialize or may not meet our projections.
We are subject to extensive governmental regulations relating to the manufacturing, labeling and marketing of our products.
Our medical technology products and operations are subject to regulation by the FDA, and other foreign and local governmental authorities. These agencies enforce laws and regulations that govern the development, testing, manufacturing, labeling, advertising, marketing and distribution, and market surveillance of our medical products.
Under the United States Federal Food, Drug, and Cosmetic Act, medical devices are classified into one of three classes — Class I, Class II or Class III — depending on the degree of risk associated with each medical device and the extent of control needed to ensure safety and effectiveness. We believe that our products currently under development and planned products will be Class II medical devices. Class II medical devices are subject to additional controls, including full applicability of the Quality System Regulations, and requirements for 510(k) pre-market notification.
The FDA may disagree with the classification of a new Class II medical device and require the manufacturer of that device to apply for approval as a Class III medical device. In the event that the FDA determines that our Class II medical Products should be classified as Class III medical devices, we could be precluded from marketing the devices for clinical use within the United States for a period of time, the length of which depends on the specific change in the classification. Reclassification of our Class II medical Products as Class III medical devices could significantly increase our regulatory costs, including the timing and expense associated with required clinical trials and other costs.
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products in foreign countries. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
The policies of the FDA and foreign regulatory authorities may change and additional government regulations may be enacted which could prevent or delay regulatory approval of our products and could also increase the cost of regulatory compliance. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
The FDA and non-U.S. regulatory authorities require that our products be manufactured according to rigorous standards. These regulatory requirements may significantly increase our production costs and may even prevent us from making our products in quantities sufficient to meet market demand. If we change our approved manufacturing process, the FDA may need to review the process before it may be used. Failure to comply with applicable regulatory requirements discussed could subject us to enforcement actions, including warning letters, fines, injunctions and civil penalties, recall or seizure of our products, operating restrictions, partial suspension or total shutdown of our production, and even criminal prosecution.
Federal, state and non-U.S. regulations regarding the manufacture and sale of medical devices are subject to future changes. The complexity, timeframes and costs associated with obtaining marketing clearances are unknown. Although we cannot predict the impact, if any, these changes might have on our business, the impact could be material.
Following the introduction of a product, these agencies will also periodically review our design and manufacturing processes and product performance. The process of complying with the applicable good manufacturing practices, adverse event reporting, clinical trial and other requirements can be costly and time consuming, and could delay or prevent the production, manufacturing or sale of our products. In addition, if we fail to comply with applicable regulatory requirements, it could result in fines, delays or suspensions of regulatory clearances, closure of manufacturing sites, seizures or recalls of products and damage to our reputation. Recent changes in enforcement practice by the FDA and other agencies have resulted in increased enforcement activity, which increases the compliance risk for the Company
15
and other companies in our industry. In addition, governmental agencies may impose new requirements regarding registration, labeling or prohibited materials that may require us to modify or re-register products already on the market or otherwise impact our ability to market our products in those countries. Once clearance or approval has been obtained for a product, there is an obligation to ensure that all applicable FDA, and other regulatory requirements continue to be met.
Additionally, injuries caused by the malfunction or misuse of cardiac devices, even where such malfunction or misuse occurs with respect to one of our competitor’s products, could cause regulatory agencies to implement more conservative regulations on the medical cardiac monitoring industry, which could significantly increase our operating costs.
If we are not able to both obtain and maintain adequate levels of third-party reimbursement for our products, it would have a material adverse effect on our business.
Healthcare providers and related facilities are generally reimbursed for their services through payment systems managed by various governmental agencies worldwide, private insurance companies, and managed care organizations. The manner and level of reimbursement in any given case may depend on the site of care, the procedure(s) performed, the final patient diagnosis, the device(s) utilized, available budget, the efficacy, safety, performance and cost-effectiveness of our planned products and services, or a combination of these or other factors, and coverage and payment levels are determined at each payer’s discretion. The coverage policies and reimbursement levels of these third-party payers may impact the decisions of healthcare providers and facilities regarding which medical products they purchase and the prices they are willing to pay for those products. Thus, changes in reimbursement levels or methods may impact sales of our products.
We have no direct control over payer decision-making with respect to coverage and payment levels for our medical device products and services. Additionally, we expect many payers to continue to explore cost-containment strategies (e.g., comparative and cost- effectiveness studies, so-called “pay-for-performance” programs implemented by various public and private payers, and expansion of payment bundling schemes such as Accountable Care Organizations, and other such methods that shift medical cost risk to providers) that may potentially impact coverage and/or payment levels for our products and services.
The ability of physicians and other providers to successfully utilize our cardiac diagnostic and monitoring solutions and successfully allow payors to reimburse for the physicians’ technical and professional fees is critical to our business because physicians and their patients will select solutions other than ours in the event that payors refuse to adequately reimburse our technical fees and physicians’ professional fees.
Changes in reimbursement practices of third-party payers could affect the demand for our products and services and our revenue levels.
The sales of our proposed products and services could depend, in part, on the extent to which healthcare providers and facilities or individual users are reimbursed by government authorities, private insurers and other third-party payers for the costs of our products or the services performed with our products. The coverage policies and reimbursement levels of third-party payers, which can vary among public and private sources and by country, may affect which products customers purchase and the prices they are willing to pay for those products and services in a particular jurisdiction. Reimbursement rates can also affect the acceptance rate of new technologies. Legislative or administrative reforms to reimbursement systems in the United States or abroad, or changes in reimbursement rates by private payers, could significantly reduce reimbursement for medical actions using the Company’s products or result in denial of reimbursement for those products, which would adversely affect customer demand or the price customers may be willing to pay for such products and services.
We may experience difficulty in obtaining reimbursement for our services from commercial payors that consider our technology to be experimental and investigational, which would adversely affect our revenue and operating results.
Many commercial payors refuse to enter into contracts to reimburse the fees associated with medical devices or services that such payors determine to be “experimental and investigational.” Commercial payors typically label medical devices or services as “experimental and investigational” until such devices or services have demonstrated product superiority evidenced by a randomized clinical trial.
16
For example, clinical trials have been performed on some mobile cardiac telemetry devices, proving higher diagnostic yield than monitoring devices and services that are already being reimbursed. Certain remaining commercial payors, however, have stated that they do not believe the data from the clinical trials justifies the removal of the experimental designation for mobile cardiac telemetry solutions. As a result, certain commercial payors may refuse to reimburse the technical and professional fees associated with cardiac monitoring solutions such as the one expected to be offered by the Company.
If commercial payors decide not to reimburse physicians or providers for their services during the utilization of our cardiac monitoring solutions, our revenue could fail to materialize or meet our projections.
Reimbursement by Medicare is highly regulated and subject to change; our failure to comply with applicable regulations could decrease our expected revenue and may subject us to penalties or have an adverse impact on our business.
The Medicare program is administered by CMS, which imposes extensive and detailed requirements on medical services providers, including, but not limited to, rules that govern how we structure our relationships with physicians, and how and where we provide our cardiac solutions. Our failure to comply with applicable Medicare rules could result in the inability of physicians to receive reimbursement as they will likely utilize our cardiac monitoring solution under the Medicare payment program.
Consolidation of commercial payors could result in payors eliminating coverage of mobile cardiac monitoring solutions or reducing reimbursement rates.
When payors combine their operations, the combined company may elect to reimburse physicians for cardiac monitoring services at the lowest rate paid by any of the participants in the consolidation. If one of the payors participating in the consolidation does not reimburse for these services at all, the combined company may elect not to reimburse at any rate. Reimbursement rates tend to be lower for larger payors. As a result, as payors consolidate, our expected average reimbursement rate may decline.
Product defects could adversely affect the results of our operations.
The design, manufacture and marketing of our hardware and software products involve certain inherent risks. Manufacturing or design defects, unanticipated use of our products, or inadequate disclosure of risks relating to the use of our products can lead to injury or other adverse events. These events could lead to recalls or safety alerts relating to our products (either voluntary or required by the FDA, or similar governmental authorities in other countries), and could result, in certain cases, in the removal of a product from the market. A recall could result in significant costs, as well as negative publicity and damage to our reputation that could reduce demand for our products. Personal injuries or deaths relating to the use of our products could also result in product liability claims being brought against us. In some circumstances, such adverse events could also cause delays in new product approvals.
Interruptions or delays in telecommunications systems or in the data services provided to us by cellular communication providers or the loss of our wireless or data services could impair the delivery of our cardiac monitoring services.
The success of our cardiac monitoring services will be dependent upon our ability to transmit, store, retrieve, process and manage data and to maintain and upgrade our data processing and communication capabilities. Our monitoring solution relies on a third-party wireless carrier to transmit data over its data network. All data sent by our monitors via this wireless data network is expected to be routed directly to healthcare providers and data centers or third-party ECG monitoring centers. We are therefore dependent upon a third party wireless carrier to provide data transmission services to us.
As we expand our commercial activities, an increased burden is expected to be placed upon our data processing systems and the equipment upon which they rely. Interruptions of our data networks, or the data networks of our wireless carrier, for any extended length of time, loss of stored data or other computer problems could have a material adverse effect on our business and operating results. Frequent or persistent interruptions in our cardiac monitoring services could cause permanent harm to our reputation and could cause current or potential users or prescribing physicians to believe that our systems are unreliable, leading them to switch to our competitors. Such interruptions could result in liability, claims and litigation against us for damages or injuries resulting from the disruption in service.
17
Our systems are also expected to be vulnerable to damage to or interruption of telecommunication services from earthquakes, floods, fires, power loss, telecommunication failures, terrorist attacks, computer viruses, break-ins, sabotage, and acts of vandalism. Despite any precautions that we may take, the occurrence of a natural disaster or other unanticipated problems could result in lengthy interruptions in these services. We do not carry business interruption insurance to protect against losses that may result from interruptions in service as a result of system failures. Moreover, the communications and information technology industries are subject to rapid and significant changes, and our ability to operate and compete is dependent on our ability to update and enhance the communication technologies used in our systems and services.
Interruptions in computing and data management cloud systems could impair the delivery of our cardiac monitoring services.
The success of our cardiac monitoring services will be dependent upon our ability to perform computing functions associated with our cardiac signal processing algorithms and data management. The diagnostic and monitoring functions rely on the uninterrupted availability of third-party cloud based computational and data management services. Availability of the cloud-based infrastructure is a critical link in our ability to deliver our services and could have a material adverse effect on our business and operating results. Furthermore, loss of data due to catastrophic events at the sites for these cloud based computer systems could cause permanent harm to our customers. These adverse events associated with unavailability of our cloud based computational infrastructure could result in liability, claims and litigation against us for damages or injuries resulting from the disruption in service.
Our systems are also expected to be vulnerable to damage or interruption in cloud computational services from earthquakes, floods, fires, power loss, technical failures, terrorist attacks, computer viruses, break-ins, sabotage, and acts of vandalism. Despite any precautions that we may take, the occurrence of a natural disaster or other unanticipated problems could result in lengthy interruptions in these services.
We could be exposed to significant liability claims if we are unable to obtain insurance at acceptable costs and adequate levels or otherwise protect ourselves against potential product liability claims.
The testing, manufacture, marketing and sale of medical devices entail the inherent risk of liability claims or product recalls. Product liability insurance is expensive and, if available, may not be available on acceptable terms at all periods of time. A successful product liability claim or product recall could inhibit or prevent the successful commercialization of our products, cause a significant financial burden on the Company, or both, which in either case could have a material adverse effect on our business and financial condition.
We require additional capital to support our present business plan and our anticipated business growth, and such capital may not be available on acceptable terms, or at all, which would adversely affect our ability to operate.
We will require additional funds to further develop our business plan. Based on our current operating plans, we plan to use $ million in capital to fund our planned operations and sales efforts necessary to commercially launch our products. We may choose to raise additional capital beyond this in order to expedite and propel growth more rapidly. We can give no assurance that we will be successful in raising any additional funds. Additionally, if we are unable to generate sufficient planned revenues from our sales and operating activities, we may need to raise additional funds, doing so through debt and equity offerings, in order to meet our expected future liquidity and capital requirements, including capital required for the development completion and introduction of our future products and technologies. Any such financing that we undertake will likely be dilutive to current stockholders.
We intend to continue to make investments to support our business growth, including patent or other intellectual property asset creation. In addition, we may also need additional funds to respond to business opportunities and challenges, including our ongoing operating expenses, protecting our intellectual property, satisfying debt payment obligations, developing new lines of business and enhancing our operating infrastructure. While we may need to seek additional funding for such purposes, we may not be able to obtain financing on acceptable terms, or at all. In addition, the terms of our financings may be dilutive to, or otherwise adversely affect, holders of our Common Stock. We may also seek to raise additional funds through arrangements with collaborators or other third parties. We may not be able to negotiate any such arrangements on acceptable terms, if at all. If we are unable to obtain additional funding on a timely basis, we may be required to curtail or terminate some or all our business plans.
18
We cannot predict our future capital needs and we may not be able to secure additional financing.
We will need to raise additional funds in the future to fund our working capital needs and to fund further expansion of our business. We may require additional equity or debt financings, collaborative arrangements with corporate partners or funds from other sources for these purposes. No assurance can be given that necessary funds will be available for us to finance our development on acceptable terms, if at all. Furthermore, such additional financings may involve substantial dilution of our stockholders or may require that we relinquish rights to certain of our technologies or products. In addition, we may experience operational difficulties and delays due to working capital restrictions. If adequate funds are not available from operations or additional sources of financing, we may have to delay or scale back our growth plans.
The results of our research and development efforts are uncertain and there can be no assurance of the commercial success of our products.
We believe that we will need to incur additional research and development expenditures to continue development of our existing proposed products as well as research and development expenditures to develop new products and services. The products and services we are developing and may develop in the future may not be technologically successful. In addition, the length of our product and service development cycle may be greater than we originally expected, and we may experience delays in product development. If our resulting products and services are not technologically successful, they may not achieve market acceptance or compete effectively with our competitors’ products and services.
If we fail to retain certain of our key personnel and attract and retain additional qualified personnel, we might not be able to pursue our growth strategy.
Our future success will depend upon the continued service of Dr. Branislav Vajdic and other members of our key management team and our technical contributors. Though no individual is indispensable, the loss of the services of these individuals could have a material adverse effect on our business, operations, revenues or prospects. We do not currently maintain key man life insurance on the lives of these individuals.
We will not be profitable unless we can demonstrate that our products can be manufactured at low prices.
To date, we have focused primarily on research and development of the first generation version of our software and hardware products, as well as other technologies we plan to introduce in our eco-system, and their proposed marketing and distribution. Consequently, we have little experience in manufacturing these products on a commercial basis. We plan to manufacture our products through third-party manufacturers. We can offer no assurance that either we or our manufacturing partners will develop efficient, automated, low-cost manufacturing capabilities and processes to meet the quality, price, engineering, design and production standards or production volumes required to successfully mass market our products, especially at the low-cost levels we require to absorb the cost of near free distribution of our products pursuant to our proposed business plan. Even if we or our manufacturing partners are successful in developing such manufacturing capability and processes, we do not know whether we or they will be timely in meeting our product commercialization schedule or the production and delivery requirements of potential customers. A failure to develop such manufacturing processes and capabilities could have a material adverse effect on our business and financial results. If we or our suppliers fail to achieve or maintain regulatory approval of manufacturing facilities, our growth could be limited and our business could be harmed.
In order to maintain compliance with FDA and other regulatory requirements, our development and manufacturing facilities must be periodically re-evaluated and qualified under a quality system to ensure they meet production and quality standards. Suppliers of components and products used to manufacture our devices must also comply with FDA regulatory requirements, which often require significant resources and subject us and our suppliers to potential regulatory inspections and stoppages. If we or our suppliers do not maintain regulatory approval for our manufacturing operations, our business could be adversely affected.
19
Our dependence on a limited number of suppliers may prevent us from delivering our devices on a timely basis.
We currently rely on a limited number of suppliers of components for our protype devices. If these suppliers became unable to provide components in the volumes needed or at an acceptable price, we would have to identify and qualify acceptable replacements from alternative sources of supply. The process of qualifying suppliers is lengthy. Delays or interruptions in the supply of our requirements could limit or stop our ability to provide sufficient quantities of devices on a timely basis or meet demand for our devices or services, which could have a material adverse effect on our business, financial condition and results of operations.
Natural disasters and other events beyond our control could materially adversely affect us.
Natural disasters or other catastrophic events may cause damage or disruption to our operations, international commerce and the global economy, and thus could have a strong negative effect on us. Our business operations are subject to interruption by natural disasters, fire, power shortages, pandemics and other events beyond our control. Such events could make it difficult or impossible for us to deliver our products and services to our customers and could decrease demand for our products and services. The World Health Organization declared the COVID-19 outbreak a pandemic. The extent of the impact of COVID-19 on our operational and financial performance will depend on certain developments, including the duration and spread of the outbreak, the impact on our customers and employees, all of which are uncertain and cannot be predicted. At this point, the overall extent to which COVID-19 may impact our financial condition or results of operations is uncertain.
The COVID-19 pandemic may negatively affect our operations.
The COVID-19 pandemic may negatively affect our operations. The COVID-19 pandemic has resulted in social distancing, travel bans and quarantine, which has limited access to our facilities, potential customers, management, support staff and professional advisors and can, in the future, impact our manufacturing supply chain. These factors, in turn, may not only impact our operations, financial condition and demand for our products but our overall ability to react in a timely manner, to mitigate the impact of this event.
Risks Related to Our Industry
The industry in which we operate is highly competitive and subject to rapid technological change. If our competitors are better able to develop and market products that are safer, more effective, less costly, easier to use, or are otherwise more attractive, we may be unable to compete effectively with other companies.
The medical technology industry is characterized by intense competition and rapid technological change, and we will face competition on the basis of product features, clinical outcomes, price, services and other factors. Competitors may include large medical device and other companies, some of which have significantly greater financial and marketing resources than we do, and firms that are more specialized than we are with respect to particular markets. Our competition may respond more quickly to new or emerging technologies, undertake more extensive marketing campaigns, have greater financial, marketing and other resources than ours or may be more successful in attracting potential customers, employees and strategic partners.
Our competitive position will depend on multiple, complex factors, including our ability to achieve regulatory clearance and market acceptance for our products, develop new products, implement production and marketing plans, secure regulatory approvals for products under development and protect our intellectual property. In some instances, competitors may also offer, or may attempt to develop, alternative systems that may be delivered without a medical device or with a medical device superior to ours. The development of new or improved products, processes or technologies by other companies may render our products or proposed products obsolete or less competitive. The entry into the market of manufacturers located in low-cost manufacturing locations may also create pricing pressure, particularly in developing markets. Our future success depends, among other things, upon our ability to compete effectively against current technology, as well as to respond effectively to technological advances or changing regulatory requirements, and upon our ability to successfully implement our marketing strategies and execute our research and development plan. Our research and development efforts are aimed, in part, at solving increasingly complex problems, as well as creating new technologies, and we do not expect that all of our projects will be successful. If our research and development efforts are unsuccessful, our future results of operations could be materially harmed.
20
We face competition from other medical device companies that focus on similar markets.
We face competition from other companies that have longer operating histories and may have greater name recognition and substantially greater financial, technical and marketing resources than us. Many of these companies also have FDA or other applicable governmental approval to market and sell their products, and more extensive customer bases, broader customer relationships and broader industry alliances than us, including relationships with many of our potential customers. Increased competition from any of these sources could result in our failure to achieve and maintain an adequate level of customers and market share to support the cost of our operations.
Unsuccessful clinical trials or procedures relating to products under development could have a material adverse effect on our prospects.
The regulatory approval process for new products and new indications for existing products requires extensive clinical trials and procedures, including early clinical experiences and regulatory studies. Unfavorable or inconsistent clinical data from current or future clinical trials or procedures conducted by us, our competitors, or third parties, or perceptions regarding this clinical data, could adversely affect our ability to obtain necessary approvals and the market’s view of our future prospects. Such clinical trials and procedures are inherently uncertain and there can be no assurance that these trials or procedures will be completed in a timely or cost- effective manner or result in a commercially viable product. Failure to successfully complete these trials or procedures in a timely and cost-effective manner could have a material adverse effect on our prospects. Clinical trials or procedures may experience significant setbacks even after earlier trials have shown promising results. Further, preliminary results from clinical trials or procedures may be contradicted by subsequent clinical analysis. In addition, results from our already completed clinical trials or procedures may not be supported by actual long-term studies or clinical experience. If preliminary clinical results are later contradicted, or if initial results cannot be supported by actual long-term studies or clinical experience, our business could be adversely affected. Clinical trials or procedures may be suspended or terminated by us, the FDA or other regulatory authorities at any time if it is believed that the trial participants face unacceptable health risks.
Intellectual property litigation and infringement claims could cause us to incur significant expenses or prevent us from selling certain of our products.
The medical device industry in which we operate is characterized by extensive intellectual property litigation and, from time to time, we might be the subject of claims by third parties of potential infringement or misappropriation. Regardless of outcome, such claims are expensive to defend and divert the time and effort of our management and operating personnel from other business issues. A successful claim or claims of patent or other intellectual property infringement against us could result in our payment of significant monetary damages and/or royalty payments, or it could negatively impact our ability to sell current or future products in the affected category and could have a material adverse effect on business, cash flows, financial condition or results of operations.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to obtaining intellectual property protections we rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We will seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We will seek to protect our confidential proprietary information, in part, by entering into confidentiality and invention or intellectual property assignment agreements with our employees and consultants. Moreover, to the extent we enter into such agreements, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed. In general, any loss of trade secret protection or other unpatented proprietary rights could harm our business, results of operations and financial condition.
21
If we are unable to protect our proprietary rights, or if we infringe on the proprietary rights of others, our competitiveness and business prospects may be materially damaged.
We have filed for and were granted a number of utility patents in the U.S as well as through PCT covering international markets. We will continue to seek patent protection for our inventions and may seek patent protection for our proprietary designs if warranted. Seeking patent protection is a lengthy and costly process, and there can be no assurance that patents will be issued from any pending applications, or that any claims allowed from existing or pending patents will be sufficiently broad or strong to protect our designs or our proprietary technology. There is also no guarantee that any patents we hold will not be challenged, invalidated or circumvented, or that the patent rights granted will provide competitive advantages to us. Our competitors have developed and may continue to develop and obtain patents for technologies that are similar or superior to our technologies. In addition, the laws of foreign jurisdictions in which we develop, manufacture or sell our products may not protect our intellectual property rights to the same extent, as do the laws the United States.
Adverse outcomes in legal disputes regarding patent and other intellectual property rights could result in the loss of our intellectual property rights, subject us to significant liabilities to third parties, require us to seek licenses from third parties on terms that may not be reasonable or favorable to us, prevent us from manufacturing, importing or selling our products, or compel us to redesign our products to avoid infringing third parties’ intellectual property. As a result, we may be required to incur substantial costs to prosecute, enforce or defend our intellectual property rights if they are challenged. Any of these circumstances could have a material adverse effect on our business, financial condition and resources or results of operations.
Dependence on our proprietary rights and failing to protect such rights or to be successful in litigation related to such rights may result in our payment of significant monetary damages or impact offerings in our product portfolios.
Our long-term success largely depends on our ability to market technologically competitive products. If we fail to obtain or maintain adequate intellectual property protection, we may not be able to prevent third parties from using our proprietary technologies or may lose access to technologies critical to our products. Also, our currently pending industrial design patent or any future patents applications may not result in issued patents, and issued patents are subject to claims concerning priority, scope and other issues.
Furthermore, to the extent we do not file applications for patents domestically or internationally, we may not be able to prevent third parties from using our proprietary technologies or may lose access to technologies critical to our products in other countries.
Enforcement of federal and state laws regarding privacy and security of patient information may adversely affect our business, financial condition or operations.
The use and disclosure of certain health care information by health care providers and their business associates have come under increasing public scrutiny. Recent federal standards under the Health Insurance Portability and Accountability Act of 1996, or HIPAA, establish rules concerning how individually-identifiable health information may be used, disclosed and protected. Historically, state law has governed confidentiality issues, and HIPAA preserves these laws to the extent they are more protective of a patient’s privacy or provide the patient with more access to his or her health information. As a result of the implementation of the HIPAA regulations, many states are considering revisions to their existing laws and regulations that may or may not be more stringent or burdensome than the federal HIPAA provisions. We must operate our business in a manner that complies with all applicable laws, both federal and state, and that does not jeopardize the ability of our customers to comply with all applicable laws. We believe that our operations are consistent with these legal standards. Nevertheless, these laws and regulations present risks for health care providers and their business associates that provide services to patients in multiple states. Because these laws and regulations are recent, and few have been interpreted by government regulators or courts, our interpretations of these laws and regulations may be incorrect. If a challenge to our activities is successful, it could have an adverse effect on our operations, may require us to forego relationships with customers in certain states and may restrict the territory available to us to expand our business. In addition, even if our interpretations of HIPAA and other federal and state laws and regulations are correct, we could be held liable for unauthorized uses or disclosures of patient information
22
as a result of inadequate systems and controls to protect this information or as a result of the theft of information by unauthorized computer programmers who penetrate our network security. Enforcement of these laws against us could have a material adverse effect on our business, financial condition and results of operations.
We may become subject, directly or indirectly, to federal and state health care fraud and abuse laws and regulations and if we are unable to fully comply with such laws, the Company could face substantial penalties.
Although not affected at this time, our operations may in the future become directly or indirectly affected by various broad state and federal health care fraud and abuse laws, including the Federal Healthcare Programs’ Anti-Kickback Statute and the Stark law, which among other things, prohibits a physician from referring Medicare and Medicaid patients to an entity with which the physician has a financial relationship, subject to certain exceptions. If our future operations are found to be in violation of these laws, we or our officers may be subject to civil or criminal penalties, including large monetary penalties, damages, fines, imprisonment and exclusion from Medicare and Medicaid program participation. If enforcement action were to occur, our business and results of operations could be adversely affected.
We may be subject to federal and state false claims laws which impose substantial penalties.
Many of the physicians and patients whom we expect to use our services will file claims for reimbursement with government programs such as Medicare and Medicaid. As a result, we may be subject to the federal False Claims Act if we knowingly “cause” the filing of false claims. Violations may result in substantial civil penalties, including treble damages. The federal False Claims Act also contains “whistleblower” or “qui tam” provisions that allow private individuals to bring actions on behalf of the government alleging that the defendant has defrauded the government. In recent years, the number of suits brought in the medical industry by private individuals has increased dramatically. Various states have enacted laws modeled after the federal False Claims Act, including “qui tam” provisions, and some of these laws apply to claims filed with commercial insurers. We are unable to predict whether we could be subject to actions under the federal False Claims Act, or the impact of such actions. However, the costs of defending claims under the False Claims Act, as well as sanctions imposed under the False Claims Act, could adversely affect our results of operations.
Risks Related To Our Units
The price of our Common Stock and Warrants may be subject to wide fluctuations.
A consistently active trading market for our Common Stock and Warrants does not exist and may not develop or be maintained. You may not be able to sell your shares quickly or at the current market price if trading in our stock is not active. You may lose all or a part of your investment. The market price of our Common Stock and Warrants may be highly volatile and subject to wide fluctuations in response to a variety of factors and risks, many of which are beyond our control. In addition to the risks noted elsewhere in this prospectus, some of the other factors affecting our stock price may include:
• variations in our operating results;
• announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments;
• announcements by third parties of significant claims or proceedings against us;
• future sales of our Common Stock or Warrants;
• any delay in our regulatory filings for our product and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings, including without limitation the FDA’s issuance of a “refusal to file” letter or a request for additional information;
• adverse results or delays in clinical trials;
• our decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial;
• adverse regulatory decisions, including failure to receive regulatory approval of our product;
23
• changes in laws or regulations applicable to our products, including but not limited to clinical trial requirements for approvals;
• adverse developments concerning our manufacturers;
• our inability to obtain adequate product supply for any approved product or inability to do so at acceptable prices;
• our inability to establish collaborations if needed;
• additions or departures of key scientific or management personnel;
• introduction of new products or services offered by us or our competitors;
• announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors;
• our ability to effectively manage our growth;
• the size and growth of our initial target markets;
• our ability to successfully treat additional types of indications or at different stages;
• actual or anticipated variations in quarterly operating results;
• our cash position;
• our failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public;
• publication of research reports about us or our industry, or positive or negative recommendations or withdrawal of research coverage by securities analysts;
• changes in the market valuations of similar companies;
• overall performance of the equity markets;
• sales of our Common Stock by us or our stockholders in the future;
• trading volume of our Common Stock;
• changes in accounting practices;
• ineffectiveness of our internal controls;
• disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our or our licensee’s technologies;
• significant lawsuits, including patent or stockholder litigation;
• general political and economic conditions; and
• other events or factors, many of which are beyond our control.
The offering price of the Units may not be indicative of the value of our assets or the price at which shares can be resold. The offering price of the Units may not be an indication of our actual value.
Prior to this offering, there has been no public market for our securities. The offering price of per Unit was determined based upon negotiations between the underwriters and us. Factors considered in determining such price in addition to prevailing market conditions include an assessment of our future prospects, an increase in value of our stock due to becoming a public company and prior valuations of our Units prepared for us. Such price does not have any relationship to any established criteria of value, such as book value or earnings per share. Such price may not be indicative of the current market value of our assets. No assurance can be given that the securities underlying our Units can be resold at the public offering price.
24
For these reasons, comparing our operating results on a period-to-period basis may not be meaningful, and you should not rely on past results as an indication of future performance. In the past, following periods of volatility in the market price of a public company’s securities, securities class action litigation has often been instituted against the public company. Regardless of its outcome, this type of litigation could result in substantial costs to us and a likely diversion of our management’s attention. You may not receive a positive return on your investment when you sell your shares and you may lose the entire amount of your investment.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our Common Stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on our Company. If no securities or industry analysts commence coverage of our Company, the trading price for our stock would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price may decline. If one or more of these analysts ceases coverage of our Company or fails to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
We may, in the future, issue additional shares of Common Stock or Warrants, which would reduce investors’ percent of ownership and dilute our share value
Our Certificate of Incorporation authorizes the issuance of 20,000,000 shares of Common Stock. As of September 30, 2021, there are outstanding 3,555,326 shares of Common Stock.
Our need for future financing may result in the issuance of additional securities which will cause investors to experience dilution.
Our cash requirements may vary from those now planned depending upon numerous factors, including the result of future research and development activities, our ability to estimate future expenses and acceptance of our products in the market.
While the proceeds derived from the sale of the shares in this offering, according to our plans, will be enough to fund commercialization of the ER Product, they will not provide us with sufficient working capital to fund commercialization of our telehealth Product. There are no commitments for future financing of the commercial phase of our telehealth Product and other future products. Though we believe a successful ER Product introduction will be a significant value creation event for us, our securities may be offered to other investors at a price lower than the price per share offered to the investors in the offering, or upon terms which may be deemed more favorable than offered hereunder. In addition, the issuance of securities in this offering as well as any future financing using our securities may dilute an investor’s equity ownership. Moreover, we may issue derivative securities, including options and/or warrants, from time to time, to procure qualified personnel or for other business reasons. The issuance of any such derivative securities, which is at the discretion of our board of directors, may further dilute the equity ownership of our stockholders, including the investors in this offering. No assurance can be given as to our ability to procure additional financing, if required, and on terms deemed favorable to us. To the extent additional capital is required and cannot be raised successfully, we may then have to limit our then current operations and/or may have to curtail certain, if not all, of our business objectives and plans.
If our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not retain a listing on Nasdaq and if the price of our Common Stock is less than $5.00, our Common Stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is
25
a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our Common Stock, and therefore shareholders may have difficulty selling their shares.
Liability of directors for breach of duty is limited under Delaware law.
Our certificate of incorporation limits the liability of directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
• breach of their duty of loyalty to us or our stockholders;
• act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
• unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or
• transaction from which the directors derived an improper personal benefit.
These limitations of liability do not apply to liabilities arising under the federal or state securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission.
Our bylaws provide that we will indemnify for our directors and officers to the fullest extent permitted by law, and may indemnify employees and other agents. Our bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding.
Upon completion of this offering, we intend to obtain a policy of directors’ and officers’ liability insurance.
We plan to enter into separate indemnification agreements with our directors and officers. These agreements, among other things, require us to indemnify our directors and officers for any and all expenses (including reasonable attorneys’ fees, retainers, court costs, transcript costs, fees of experts, witness fees, travel expenses, duplicating costs, printing and binding costs, telephone charges, postage, delivery service fees) judgments, fines and amounts paid in settlement actually and reasonably incurred by such directors or officers or on his or her behalf in connection with any action or proceeding arising out of their services as one of our directors or officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request provided that such person follows the procedures for determining entitlement to indemnification and advancement of expenses set forth in the indemnification agreement. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability and indemnification provisions in our certificate of incorporation and bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might provide a benefit to us and our stockholders. Our results of operations and financial condition may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
At present, there is no pending litigation or proceeding involving any of our directors or officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
We do not anticipate paying any cash dividends on our Common Stock in the foreseeable future and, as such, capital appreciation, if any, of our Common Stock will be your sole source of gain for the foreseeable future.
We do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. In addition,
26
and any future loan arrangements we enter into may contain, terms prohibiting or limiting the amount of dividends that may be declared or paid on our Common Stock. As a result, capital appreciation, if any, of our Common Stock will be your sole source of gain for the foreseeable future.
Risks Related to this Offering
We are an “emerging growth company,” and any decision on our part to comply with certain reduced disclosure requirements applicable to emerging growth companies could make our Units less attractive to investors.
We are an “emerging growth company” as defined in the JOBS Act, and, for as long as we continue to be an emerging growth company, we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, not being required to comply with any new requirements adopted by the Public Company Accounting Oversight Board (the “PCAOB”), requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer, not being required to comply with any new audit rules adopted by the PCAOB after April 5, 2012 unless the SEC determines otherwise, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could remain an emerging growth company until the earlier of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of our first sale of common equity securities pursuant to an effective registration statement; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer. We cannot predict if investors will find our Units less attractive if we choose to rely on these exemptions. If some investors find our Units less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our Units and our stock price may be more volatile. Further, as a result of these scaled regulatory requirements, our disclosure may be more limited than that of other public companies and you may not have the same protections afforded to shareholders of such companies.
Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the “Securities Act”), for complying with new or revised accounting standards. We have opted for taking advantage of the extended transition period for complying with new or revised accounting standards pursuant to Section 107(b) of the Jobs Act.
As a result of our becoming a public company, we will become subject to additional reporting and corporate governance requirements that will require additional management time, resources and expense.
As a public company, and particularly after we are no longer an emerging growth company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of the NASDAQ and other applicable securities rules and regulations impose various requirements on public companies, including the obligation to file with the SEC annual and quarterly information and other reports that are specified in the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and to establish and maintain effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
We are evaluating these rules and regulations, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
27
We have identified weaknesses in our internal controls, and we cannot provide assurances that these weaknesses will be effectively remediated or that additional material weaknesses will not occur in the future.
As a public company, we will be subject to the reporting requirements of the Exchange Act, and the Sarbanes-Oxley Act. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting and financial compliance costs, make some activities more difficult, time consuming and costly, and place significant strain on our personnel, systems and resources.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures, and internal control over financial reporting.
We do not yet have effective disclosure controls and procedures, or internal controls over all aspects of our financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we will file with the SEC is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. Our management has deemed certain conditions to be material weaknesses and significant deficiencies in our internal controls. For example, we failed to employ a sufficient number of staff to maintain optimal segregation of duties and to provide optimal levels of oversight. Our management is responsible for establishing and maintaining adequate internal control over our financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. We will be required to expend time and resources to further improve our internal controls over financial reporting, including by expanding our staff. However, we cannot assure you that our internal control over financial reporting, as modified, will enable us to identify or avoid material weaknesses in the future.
Our current controls and any new controls that we develop may become inadequate because of changes in conditions in our business, including increased complexity resulting from our international expansion. Further, weaknesses in our disclosure controls or our internal control over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls, or any difficulties encountered in their implementation or improvement, could harm our operating results or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods. Any failure to implement and maintain effective internal control over financial reporting could also adversely affect the results of management reports and independent registered public accounting firm audits of our internal control over financial reporting that we will eventually be required to include in our periodic reports that will be filed with the SEC. Ineffective disclosure controls and procedures, and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the market price of our Common Stock.
We are not currently required to comply with the SEC rules that implement Section 404 of the Sarbanes-Oxley Act, and are therefore not required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. As a public company, we will be required to provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second annual report on Form 10-K. Our independent registered public accounting firm is not required to audit the effectiveness of our internal control over financial reporting until after we are no longer an “emerging growth company” as defined in the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our internal control over financial reporting is documented, designed or operating.
Any failure to maintain effective disclosure controls and internal control over financial reporting could have a material and adverse effect on our business and operating results, and cause a decline in the market price of our Common Stock.
Future sales of a substantial number of our Common Stock by our existing shareholders could cause our stock price to decline.
We will have a significant number of restricted Common Stock that will become eligible for sale shortly after this registration statement is declared effective. Prior to the consummation of this offering on a pro forma basis we will have 3,555,326 shares of our Common Stock outstanding. Upon consummation of this offering we have agreed to issue 2,750,000 shares of our Common Stock based on an assumed initial public offering price of $6.00 per share of Common Stock and convertible notes that convert to 1,449,574 shares of Common Stock (based on a conversion price of $4.20/share and interest calculated through September 30, 2021). All of the shares sold in this offering will be eligible for sale immediately upon effectiveness of this registration statement. All of the remaining shares, including the shares issued upon conversion of the convertible notes, will be eligible for sale in the public market upon expiration
28
of lock-up agreements in 180 days after the date of this prospectus. It is conceivable that following the holding period, many shareholders may wish to sell some or all of their shares. If our shareholders sell substantial amounts of our Common Stock in the public market at the same time, the market price of our Common Stock could decrease significantly due to an imbalance in the supply and demand of our Common Stock. Even if they do not actually sell the Common Stock, the perception in the public market that our shareholders might sell significant Common Stock could also depress the market price of our Common Stock.
Future sales and issuances of our Common Stock or rights to purchase Common Stock, including pursuant to our equity incentive plans and outstanding warrants could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital may be needed in the future to continue our planned operations, including conducting clinical trials, commercialization efforts, expanded research and development activities and costs associated with operating a public company. To raise capital, we may sell Common Stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell Common Stock, convertible securities or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to the holders of our Common Stock, including shares of Common Stock sold in this offering. Initially, the aggregate number of shares of our Common Stock that may be issued pursuant to stock awards under our 2015 Plan is approximately 1,636,000 shares. Increases in the number of shares available for future grant or purchase may result in additional dilution, which could cause our stock price to decline. In addition, the issuance of shares of Common Stock upon conversion of the 2015 Convertible Notes, if converted at the option of the holder, will also have a dilutive effect on the percentage ownership held by holders of our Common Stock.
The Company will have broad discretion in the use of the net proceeds from this offering and may fail to apply these proceeds effectively.
The Company’s management will have broad discretion in the application of the net proceeds of this offering, including using the proceeds to conduct operations, expand the Company’s business lines and for general working capital. The Company may also use the net proceeds of this offering to acquire or invest in complementary businesses, products, or technologies, or to obtain the right to use such complementary technologies. We have no commitments with respect to any acquisition or investment; however, we seek opportunities and transactions that management believes will be advantageous to the Company and its operations or prospects. We cannot specify with certainty the actual uses of the net proceeds of this offering. You may not agree with the manner in which our management chooses to allocate and spend the net proceeds. We may invest the net proceeds from this offering in a manner that does not produce income or that loses value. The failure by our management to apply these funds effectively could harm our business, financial condition and results of operations.
There is no assurance that an active and liquid trading market in our Common Stock and Warrants will develop.
This offering will close only if our Common Stock and Warrants accepted to be listed on the Nasdaq Capital Market. There can be no assurance any broker will be interested in trading our Common Stock or Warrants. Therefore, it may be difficult to sell any securities you purchase in this offering if you desire or need to sell them. Neither we nor the underwriters can provide any assurance that an active and liquid trading market in our Common Stock or Warrants will develop or, if developed, that the market will continue.
There is no guarantee that we will successfully have our Common Stock and Warrants listed on the Nasdaq Capital Market. Even if our Common Stock and Warrants are accepted for listing on the Nasdaq Capital Market, upon our satisfaction of the exchange’s initial listing criteria, the exchange may subsequently delist our Common Stock if we fail to comply with ongoing listing standards.
In the event we are able to list our Common Stock and Warrants on the Nasdaq Capital Market upon our satisfaction of the exchange’s initial listing criteria, the exchange will require us to meet certain financial, public float, bid price and liquidity standards on an ongoing basis in order to continue the listing of our Common Stock and Warrants. If we fail to meet these continued listing requirements, our Common Stock or Warrants may be subject to delisting. If our Common Stock or Warrants are delisted and we are not able to list such Common Stock and Warrants on another national securities exchange, we expect our securities would be quoted on an over-the-counter market; However, if this
29
were to occur, our stockholders could face significant material adverse consequences, including limited availability of market quotations for our Common Stock and Warrants and reduced liquidity for the trading of our securities. In addition, in the event of such delisting, we could experience a decreased ability to issue additional securities and obtain additional financing in the future. Even if our Common Stock and Warrants are listed on the Nasdaq Capital Market, there can be no assurance that an active trading market for our Common Stock or Warrants will develop or be sustained after our initial listing.
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
If you purchase shares of Common Stock in this offering, the value of your shares based on our actual book value will immediately be less than the price you paid. This reduction in the value of your equity is known as dilution. This dilution occurs in large part because our existing stockholders paid less than the assumed public offering price when they acquired their shares of Common Stock. Based upon the issuance and sale of 2,750,000 shares of Common Stock by us in this offering at an assumed public offering price of $6.00 per share, you will incur immediate dilution of in the net tangible book value per share of Common Stock. If the underwriters exercise their over-allotment option, or if outstanding options to purchase our Common Stock are exercised, investors will experience additional dilution. For more information, see “Dilution.”
30
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. These forward-looking statements contain information about our expectations, beliefs or intentions regarding our product development and commercialization efforts, business, financial condition, results of operations, strategies or prospects, and other similar matters. These forward-looking statements are based on management’s current expectations and assumptions about future events, which are inherently subject to uncertainties, risks and changes in circumstances that are difficult to predict. These statements may be identified by words such as “expects,” “plans,” “projects,” “will,” “may,” “anticipates,” “believes,” “should,” “intends,” “estimates,” and other words of similar meaning.
These statements relate to future events or our future operational or financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the section titled “Risk Factors” and elsewhere in this prospectus, in any related prospectus supplement and in any related free writing prospectus.
Any forward-looking statement in this prospectus, in any related prospectus supplement and in any related free writing prospectus reflects our current view with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our business, results of operations, industry and future growth. Given these uncertainties, you should not place undue reliance on these forward-looking statements. No forward-looking statement is a guarantee of future performance. You should read this prospectus, any related prospectus supplement and any related free writing prospectus and the documents that we reference herein and therein and have filed as exhibits hereto and thereto completely and with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
This prospectus, any related prospectus supplement and any related free writing prospectus also contain or may contain estimates, projections and other information concerning our industry, our business and the markets for our products, including data regarding the estimated size of those markets and their projected growth rates. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry and general publications, government data and similar sources. In some cases, we do not expressly refer to the sources from which these data are derived.
31
Assuming the sale of all of the Units in this offering at an assumed offering price of $6 per share, the Company estimates that the net proceeds from the sale of shares it is offering will be approximately $14,695,000. If the underwriters fully exercise the over-allotment option, the net proceeds will be approximately $16,996,750. “Net proceeds” is what the Company expects to receive after deducting the underwriting discount and commission and estimated offering expenses payable by the Company.
The Company intends to use the net proceeds from this offering to conduct operations, increase marketing efforts, and investments in the Company’s existing business initiatives and products, as well as general working capital. The Company anticipates budgeting approximately $14.7 million of the proceeds from the offering for conducting operations and for working capital.
The Company may also use a portion of the net proceeds of this offering to acquire or invest in complementary businesses, products, or technologies, or to obtain the right to use such complementary technologies. The Company has no commitments with respect to any acquisition or investment and is not currently involved in any negotiations with respect to any such transactions.
We currently intend to use the net proceeds from this offering as follows:
• approximately $1,700,000 to fund our initial product, the ER product, including the achievement of FDA 510(k) clearance and commercial launch in the third quarter of 2022;
• approximately $7,000,000 to fund engineering and regulatory work for our telehealth product, to achieve FDA 510(k) submission of the telehealth product in the fourth quarter of 2022 and to ready the product for limited market release in the second quarter of 2023; and
• the balance for working capital and general corporate purposes.
This expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions.
As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. The amounts and timing of its actual expenditures will depend on numerous factors, including the status of its product development efforts, sales and marketing activities, technological advances, amount of cash generated or used by its operations and competition. Accordingly, our management will have broad discretion in the application of the net proceeds and investors will be relying on the judgment of its management regarding the application of the proceeds of this offering.
We believe we will need to raise additional funding of approximately $20 million for market launch of our telehealth Product and ongoing development and commercialization of additional telehealth Products before becoming cash flow positive.
32
The Company has not declared nor paid any cash dividend on its Common Stock, and it currently intends to retain future earnings, if any, to finance the expansion of its business, and the Company does not expect to pay any cash dividends in the foreseeable future. The decision whether to pay cash dividends on its Common Stock will be made by its board of directors, in their discretion, and will depend on the Company’s financial condition, results of operations, capital requirements and other factors that its board of directors considers significant.
33
The following table sets forth the Company’s cash and capitalization as of June 30, 2021 on:
• an actual basis;
• on a pro forma basis to give effect to the conversion of outstanding 2015 notes plus interest into 1,263,571 shares of Common Stock; and
• a pro forma, as adjusted basis giving further effect to the sale and issuance by the Company of shares of Common Stock being sold in this offering at assumed the public offering price of $6.00 per share, resulting in net proceeds to the Company of $14,695,000 after deducting underwriting discounts and commissions and estimated offering expenses payable by the Company.
The information in this table is unaudited and is illustrative only and the Company’s capitalization following the completion of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with the information contained in “Use of Proceeds,” “Summary Financial Information” and “Management’s Discussion and Analysis of Financial Condition and Results of Operation,” as well as the financial statements and the notes included elsewhere in this prospectus.
|
(In thousands, except share data) |
June 30, |
Conversion of debt |
Proceeds |
Adjusted |
|||||||||||
|
(Unaudited) |
|||||||||||||||
|
Cash |
$ |
465 |
|
$ |
0 |
|
$ |
14,695 |
$ |
15,160 |
|
||||
|
Convertible Debt, net |
|
4,194 |
|
|
(4,194 |
) |
|
0 |
|
0 |
|
||||
|
Stockholders’ (Deficit) Equity |
|
|
|
|
|
|
|
||||||||
|
Common stock par value $0.0001: 20,000,000 shares authorized; 3,547,168 shares issued and outstanding, pro forma; 7,560,753 shares issued and outstanding as adjusted |
|
0 |
|
|
0 |
|
|
1 |
|
1 |
|
||||
|
Additional paid in capital |
|
1,687 |
|
|
4,194 |
|
|
14,694 |
|
20,575 |
|
||||
|
Accumulated deficit |
|
(5,951 |
) |
|
0 |
|
|
0 |
|
(5,951 |
) |
||||
|
Total (Deficit) Equity |
|
(4,264 |
) |
|
4,194 |
|
|
14,695 |
|
14,625 |
|
||||
|
Capitalization |
|
(70 |
) |
|
0 |
|
|
14,695 |
|
14,625 |
|
||||
Each $1.00 increase (decrease) in the assumed public offering price of $6.00 per share would increase (decrease) the pro forma as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders’ equity (deficit) by approximately $2.6 million assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 100,000 shares in the number of shares offered by us at the assumed public offering price of $6.00 per share would increase (decrease) the pro forma as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders’ equity (deficit) by approximately $558,000.
The number of shares of Common Stock outstanding is based on 3,547,168 shares of Common Stock issued and outstanding as of June 30, 2021, and excludes the following:
• 729,242 shares of common stock issuable upon the exercise of outstanding stock options at a weighted average exercise price of $0.94 per share;
• 422,549 shares of common stock issuable upon the exercise of outstanding warrants at a weighted average exercise price of $0.11 per unit; and
• 656,666 shares of common stock available for future issuance under the 2015 Equity Incentive Plan.
34
If you invest in the Company’s Units in this offering, your ownership interest will be diluted to the extent of the difference between the assumed offering price per share of its Common Stock (giving no value to the Warrants) and the as adjusted net tangible book value per share of its Common Stock immediately after the offering. Historical net tangible book value per share represents the amount of the Company’s total tangible assets less total liabilities, divided by the number of shares of its Common Stock outstanding.
The historical net tangible book value (deficit) of the Company’s Common Stock as of June 30, 2021, was approximately $(4.3 million) or $(1.20) per share based upon shares of Common Stock outstanding on such date. Historical net tangible book value (deficit) per share represents the amount of its total tangible assets reduced by the amount of its total liabilities, divided by the total number of shares of Common Stock outstanding. The pro forma historical net tangible book value (deficit) was $(0.02) per share based upon shares of Common Stock outstanding including the conversion of the Convertible Notes (and interest) through a Qualified Financing such as this IPO, at a conversion price equal to seventy (70)% of the price per share in the public offering (as defined in the Convertible Notes).
After giving effect to the Company’s sale of all of the 2,750,000 shares of Common Stock offered in this offering (giving no value to the Warrants) at an assumed public offering price of $6.00 per share after deducting estimated underwriting discounts and commissions and the Company’s estimated offering expenses, and after the Company’s pro forma as adjusted net tangible book value as of June 30, 2021 would have been $14,625,000 or $1.93 per share. This represents an immediate increase in net tangible book value of $1.95 per share to the Company’s existing stockholders, and an immediate dilution in net tangible book value of $4.07 per share to new investors. The following table illustrates this per share dilution:
|
Assumed public offering price per share |
$ |
6.00 |
|
|
|
Historical net tangible book value (deficit) per share as of June 30, 2021 |
$ |
(1.20 |
) |
|
|
Pro forma historical net tangible book value (deficit) per share as of June 30, 2021 attributable to the pro forma transaction described above |
$ |
(0.02 |
) |
|
|
Increase in pro forma net tangible book value per share as of June 30, 2021 attributable to the pro forma transactions described above |
$ |
1.95 |
|
|
|
Pro forma net tangible book value per share as of June 30, 2021 |
$ |
1.93 |
|
|
|
Dilution per share to new investors in this offering |
$ |
4.07 |
|
The information discussed above is illustrative only, and the dilution information following this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. A $1.00 increase (decrease) in the assumed public offering price of $6.00 per share would increase (decrease) the pro forma as adjusted net tangible book value by $0.39 per share and increase the dilution to new investors by $0.60 per share and decrease the dilution to new investors by $0.61 per share, assuming the number of shares offered by the Company, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by the Company. The Company may also increase or decrease the number of shares it is offering. An increase of 100,000 shares offered by it would increase the pro forma as adjusted net tangible book value by $0.05 per share and decrease the dilution to new investors by $0.05 per share, assuming the assumed public offering price of $6.00 per share remains the same and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by the Company. Similarly, a decrease of 100,000 shares offered by the Company would decrease the pro forma as adjusted net tangible book value by $0.04 per share and increase the dilution to new investors by $0.04 per share, assuming the assumed public offering price of $6.00 per share remains the same and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by the Company.
If the underwriters’ over-allotment option to purchase additional shares from the Company is exercised in full, and based on the assumed public offering price of $6.00 per share, the pro forma as adjusted net tangible book value per share after this offering would be $2.14 per share, the increase in as adjusted net tangible book value per share to existing stockholders would be $2.12 per share and the dilution to new investors purchasing shares in this offering would be $3.88 per share.
35
The number of shares of Common Stock outstanding is based on 3,547,168 shares of Common Stock issued and outstanding as of June 30, 2021, and excludes the following:
• 729,242 shares of Common Stock issuable upon the exercise of outstanding stock options having a weighted average exercise price of $0.94 per share;
• 422,549 shares of Common Stock issuable upon the exercise of outstanding warrants having a weighted average exercise price of $0.11 per share;
• 656,666 shares of Common Stock reserved for future issuance under the Company’s 2015 Equity Incentive Plan (the “2015 Plan”).
Except as otherwise indicated herein, all information in this prospectus assumes:
• no exercise of the outstanding options or warrants described above; and
• no exercise of the underwriters’ option to purchase up to an additional 412,500 shares of Common Stock to cover over-allotments, if any, or any warrants issued to the underwriters as fees.
36
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the financial statements and related notes to the financial statements included elsewhere in this prospectus. This discussion contains forward-looking statements that relate to future events or our future financial performance. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. These risks and other factors include, among others, those listed under “forward-looking statements” and “risk factors” and those included elsewhere in this prospectus. All share and per share numbers have been retroactively adjusted to reflect the 1-for-2.75 reverse stock split effected on September 27, 2021.
Overview
We are a development stage medical technology company with the primary focus on cutting edge telemedicine solutions that enable cardiac disease detection and monitoring outside of a healthcare facility setting. Our aim is to deliver innovative, remote diagnostic and monitoring technologies for the benefit of patients, healthcare providers and healthcare payers.
Cardiovascular disease is the number one cost to the healthcare system and is estimated to be responsible for 1 in every 6 healthcare dollars spent in the US. Since cardiovascular disease is the leading cause of death worldwide, early detection, diagnosis, and management of chronic cardiac conditions are critical to reducing the increasing burden on the healthcare infrastructure. Diagnostic tests such as ECGs are used to detect, diagnose and track numerous cardiovascular conditions. We believe that the trend of moving diagnostic data collection and care for heart disease to the home is a permanent change. This trend will continue to place increased emphasis on cost-effective diagnostic and monitoring technologies in the ECG market.
Our initial telemedicine technology Product will address the heart attack detection market as well as the market to monitor high-risk coronary artery disease (CAD). Currently there are no products on the market that are simple to use and easy to carry with the ability to provide physicians and patients with timely and highly accurate information about potential Acute Coronary Syndrome (ACS) and Myocardial Infarction (MI) events.
There are two major markets for our products: remote patient monitoring and the hospital Emergency Room (ER). We believe that we are uniquely positioned to play a central role in remote monitoring of the high-risk coronary artery disease patients, a well-defined need that we believe has not been addressed adequately. Remote patient monitoring of cardiac patients is a multi-billion dollar market and promises to increase patient compliance and reduce healthcare costs. We believe that our solution fits with established CPT codes for remote patient monitoring. Our long-term strategy is to undertake clinical studies demonstrating the benefits of the system on outcomes and healthcare costs, and to use the clinical data to establish coverage and payment specific to our system. Secondly, we are applying our platform technology to create a software tool for detecting a heart attack in the ER environment. This software tool is designed to increase the emergency physician’s confidence in diagnosing heart attacks. It will be our initial commercial offering, as it has reduced complexities, compared to the full telehealth solution, and it provides the shortest path to revenue. This Product will be offered on a software licensing basis.
To date, we have developed working prototypes for both our telehealth Product and our ER Product. The ER Product is currently undergoing additional engineering work that we believe will make it ready for the FDA 510(k) clearance submission. Both Products have been validated in three medical studies that were designed by Harvard Medical School faculty. Peer reviewed publications that describe the studies and results are in preparation. One peer reviewed medical publication submission is planned for September 2021 and another for November 2021. These two publications will describe results of our two key studies: HIDES and B Score. In early 2022 we plan to publish results of a study on performance of our ER Product in the real-life environment of an ER. In July 2021 we submitted a technology abstract for presentation at the IEEE EMBS 2021 Conference; it was accepted. The abstract describes the technology foundation of our ER Product.
We believe that we have a very strong intellectual property position. All intellectual property rights associated with Company’s technology are owned by the Company.
Our focus over next 18 months will be on getting FDA clearances for both of our products and introducing our first Products to the market. We believe that both Products will be subject to the FDA 510(k) review process. The predicate devices have been identified. We are partnering with Ximedica, a leading medical device design and manufacturing firm, to finalize the development of our initial Products to meet all FDA requirements for the 510(k) submission.
37
Significant Developments in early 2021
On September 27, 2021, the Company filed a certificate of amendment to its amended and restated certificate of incorporation with the Secretary of State of the State of Delaware to effect a 1-for-2.75 reverse stock split of its outstanding shares of common stock. As a result of the reverse stock split, every 2.75 shares of the Company’s outstanding pre-reverse split common stock were combined and reclassified into one share of common stock.
We have raised an additional $1,715,000 in 2021 from the issuance of additional 2015 Notes. The Board has authorized up to $5.5 million in these notes, of which $416,000 remain available for sale as of the date of this registration statement.
On March 22, 2021, we amended the definition of Qualified Financing in the 2015 Convertible Notes to include common shares as well as preferred stock. The 2015 Notes accrue interest payable at the rate of eight percent (8%) and the conversion price is equal to seventy percent (70%) of the per share price at which shares of stock is to be sold in a Qualified Financing, which includes the shares sold under this registration statement. They otherwise mature December 31, 2021.
All our Directors and Officers have invested in our 2015 Notes, as have several consultants who provide services. As of June 30, 2021, investments from Directors and Officers represents approximately $2,308,000; investments from consultants represents approximately $886,000. The remaining investors are individual accredited investors.
In June 2021, we appointed Richard Ferrari as our Executive Chairman. In September 2021 we appointed George A. de Urioste to the Board of Directors and Jon Hunt, PhD as Executive Vice President and Chief Business Officer. Wim Elfrink remains on the Board.
A significant market opportunity exists for a 12-lead ECG patch. We have received a notice that our US patent application No.17/202,299 was issued. Our patented 12-lead patch future product has potential to disrupt the large and growing market for ECG patches. All patches that are currently in the market are single lead ECG patches. The medical value of providing to a physician a 12-lead ECG for a symptomatic event is much higher when compared to that of a single lead patch.
An initial clinical study which was completed on the HIDES database in early 2021 indicates that our ER software offering could offer considerable improvement in the accuracy of MI detection compared to a panel of experienced cardiologists interpreting a standard 12 lead ECG. The importance of increased accuracy of the first ECG interpretation for a chest pain patient presenting to an ER is great, potentially leading to faster intervention or avoiding unnecessary activation of the cardiac catheterization lab.
Results of Operations
We operate a medical device business. The following table summarizes our results of operations for the periods presented and as a percentage of our total revenue for those periods based on our consolidated statement of operations data. The year over year comparison of results of operations is not necessarily indicative of results of operations for future periods.
Summary of Statements of Operations for the three and six months ended June 30, 2021 compared with the three and six months ended June 30, 2020:
|
For Three Months ended June 30, |
For the Six Months ended June 30, |
|||||||||||||||||||||||
|
2021 |
2020 |
Change |
% Change |
2021 |
2020 |
Change |
% Change |
|||||||||||||||||
|
(In thousands, except percentages) |
||||||||||||||||||||||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
||||||||||||||||
|
General and administrative |
312 |
|
133 |
|
179 |
|
135 |
% |
446 |
|
227 |
|
219 |
|
96 |
% |
||||||||
|
Research and development |
25 |
|
21 |
|
4 |
|
19 |
% |
54 |
|
28 |
|
26 |
|
93 |
% |
||||||||
|
Total operating expenses |
337 |
|
154 |
|
183 |
|
119 |
% |
500 |
|
255 |
|
245 |
|
96 |
% |
||||||||
|
Loss from operations |
(337 |
) |
(154 |
) |
(183 |
) |
119 |
% |
(500 |
) |
(255 |
) |
(245 |
) |
96 |
% |
||||||||
|
Interest expense |
(608 |
) |
(75 |
) |
(533 |
) |
711 |
% |
(677 |
) |
(147 |
) |
(530 |
) |
361 |
% |
||||||||
|
Other income |
— |
|
— |
|
— |
|
— |
|
22 |
|
— |
|
22 |
|
100 |
% |
||||||||
|
Income tax provision |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
||||||||
|
Net loss |
(945 |
) |
(229 |
) |
(716 |
) |
391 |
% |
(1,155 |
) |
(402 |
) |
(753 |
) |
187 |
% |
||||||||
38
Research and developments expenses (“R&D”) are primarily from internally developed software and our credit-card sized collection device. Our focus on R&D consisted largely of professional services associated with the development of our telehealth system and in 2021 the initial development work on the software-only ER Product.
General and administrative expenses (“G&A”) are largely related to personnel and professional services. During the three and six months ended June 30, 2021, G&A expense increased $179,000 or 135% and $219,000 or 96%, respectively when compared to the same periods in 2020. The primary increases were in business development, which included public company readiness expenses.
Interest expense increased $533,000 and $530,000 during the three and six months ended June, 2021, when compared to 2020, and is primarily due to the accretion of the Convertible Notes 30% discount of $531,000 during the quarter ending June 30, 2021.
Other income increased $22,000 for the six month ended June 30, 2021 due to the forgiveness of loans during the first quarter of 2021 issued under the CARES Act.
Summary of Statements of Operations for the Years ended December 31, 2020 compared with the year ended December 31, 2019:
|
For the Years Ended December 31, |
|||||||||||||||
|
2020 |
2019 |
Change |
% Change |
||||||||||||
|
(In thousands, except percentages) |
|||||||||||||||
|
Operating expenses: |
|
|
|
|
|
|
|
||||||||
|
General and administrative |
$ |
655 |
|
$ |
253 |
|
$ |
402 |
|
159 |
% |
||||
|
Research and development |
|
133 |
|
|
41 |
|
|
92 |
|
224 |
% |
||||
|
Total operating expenses |
|
788 |
|
|
294 |
|
|
494 |
|
168 |
% |
||||
|
Loss from operations |
|
(788 |
) |
|
(294 |
) |
|
(494 |
) |
168 |
% |
||||
|
Interest expense |
|
(280 |
) |
|
(242 |
) |
|
(38 |
) |
16 |
% |
||||
|
Income tax provision |
|
— |
|
|
— |
|
|
— |
|
— |
|
||||
|
Net loss |
$ |
(1,068 |
) |
$ |
(536 |
) |
$ |
(532 |
) |
99 |
% |
||||
During the year ended December 31, 2020 R&D expense increased $92,000 when compared to 2019, an increase of 224%, as we advanced towards the FDA clearance of the first product release.
During the year ended December 31, 2020, G&A expense increased $402,000 or 159% when compared to 2019. The primary increases were in business development, which included the development of our reimbursement strategy, and public company readiness expenses.
Interest expense increased $38,000 in during the year-ended December 31, 2020 when compared to 2019, primarily due to interest from issuance of additional 8% convertible notes in 2020.
Liquidity and Capital Resources
Our cash requirements are and will continue to be, dependent upon a variety of factors. We expect to continue devoting significant capital resources to R&D and go to market strategies.
As of June 30, 2021, we had approximately $465,000 of cash, an increase of $441,000 from $24,000 as of December 31, 2020.
During the six months ended June 30, 2021, we raised and additional $865,000 in 2015 Notes to support our on-going business plan.
39
Our cash is as follows (in thousands):
|
June 30, |
December 31, |
|||||
|
Cash |
$ |
465 |
$ |
24 |
||
Cash flows for the six months ended June 30, 2021 and 2020 (in thousands):
|
Six Months ended June 30 |
||||||||
|
2021 |
2020 |
|||||||
|
Net cash used in operating activities |
$ |
(424 |
) |
$ |
(167 |
) |
||
|
Net cash provided by financing activities |
$ |
865 |
|
$ |
293 |
|
||
Operating Activities:
Net cash used by our operating activities of $424,000 during the six months ended June 30, 2021, is primarily due to our net loss of $1,155,000 less $688,000 in non-cash expenses, offset by a decrease of $43,000 of net changes in operating assets and liabilities.
Net cash used by our operating activities of $167,000 during the six months ended June 30, 2020, is primarily due to our net loss of $402,000 less $147,000 in non-cash interest expense, stock based compensation and amortization of debt issuance costs, offset by a decrease of $88,000 from changes in operating assets and liabilities.
Financing Activities:
During the six months ended June 30, 2021, and 2020 net cash provided by financing activities was $865,000 and $293,000, respectively, and is primarily from the issuance of our 2015 Notes.
We received a PPP loan and EIDL grant during the six months ended June 30, 2020 for a total value of approximately $23,000, both were forgiven in the first quarter of 2021.
Our cash is as follows (in thousands):
|
2020 |
2019 |
|||||
|
Cash |
$ |
24 |
$ |
5 |
||
Cash flows for the year ended December 31, 2020 and 2019 (in thousands):
|
2020 |
2019 |
|||||||
|
Net cash used in operating activities |
$ |
(600 |
) |
$ |
(218 |
) |
||
|
Net cash provided by financing activities |
$ |
619 |
|
$ |
206 |
|
||
Operating Activities:
Net cash used by our operating activities of $600,000 during the twelve months ended December 31, 2020, is primarily due to our net loss of $1,068,000 less $286,000 in non-cash interest expense, non-cash stock-based compensation and amortization of debt issuance costs, offset by $182,000 of net changes in operating assets and liabilities.
Net cash used by our operating activities of $218,000 during the twelve months ended December 31, 2019, is primarily due to our net loss of $536,000 less $242,000 in non-cash interest expense and amortization of debt issuance costs, offset by $76,000 of net changes in operating assets and liabilities.
Financing Activities:
During the year ended December 31, 2020 net cash provided by financing activities was $619,000 and was primarily from the issuance of our 2015 convertible notes.
40
During the year ended December 31, 2019 net cash provided by financing activities was $206,000 and was primarily from the issuance of our 2015 convertible notes and short-term loans, a majority of which were converted to the 2015 notes.
Off-Balance Sheet Arrangements
None
Critical Accounting Policies and Estimates
We believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating this “Management’s Discussion and Analysis of Financial Condition and Results of Operation.”
The discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or “U.S. GAAP.” The preparation of these financial statements in accordance with U.S. GAAP requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, assumptions and judgments, including those related to revenue recognition, bad debts, inventories, warranties and income taxes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and our revenue recognition. Actual results may differ from these estimates under different assumptions or conditions and the impact of such differences may be material to our consolidated financial statements.
Critical accounting policies are those policies that, in management’s view, are most important in the portrayal of our financial condition and results of operations. The methods, estimates and judgments that we use in applying our accounting policies have a significant impact on the results that we report in our financial statements. These critical accounting policies require us to make difficult and subjective judgments, often as a result of the need to make estimates regarding matters that are inherently uncertain. Those critical accounting policies and estimates that require the most significant judgment are discussed further below. We consider our most critical accounting policies and estimates to be revenue recognition, gain on settlements, valuation of long lived assets, income taxes and valuation allowances against net deferred tax assets, derivative liabilities, stock-based compensation and accounting for business combinations-acquisition method accounting.
Stock-based compensation payments to employees and consultants are recognized as expense in the statements of income. The compensation cost for all stock-based awards is measured at the grant date, based on the fair value of the award (determined using a Black-Scholes option pricing model for stock options and intrinsic value on the date of grant for non-vested restricted stock), and is recognized as an expense over the employee’s requisite service period (generally the vesting period of the equity award). Determining the fair value of stock-based awards at the grant date requires significant estimates and judgments, including estimating the market price volatility of our Common Stock, future employee stock option exercise behavior and requisite service periods.
Stock-based compensation expense is recorded only for those awards expected to vest using actual forfeitures. The estimation of stock awards that will ultimately vest requires judgment, and to the extent that actual results differ from our estimates, such amounts will be recorded as cumulative adjustments in the period the estimates are revised.
Determination of Fair Value of Common Stock
As there has been no public market for our Common Stock to date, the estimated fair value of our Common Stock has been determined by our Board of Directors as of the date of each option grant, with input from management, considering the most recently available third-party valuations of our Common Stock and our Board of Directors’ assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent valuation through the date of the grant.
41
Given the absence of a public trading market of our Common Stock, and in accordance with the guidance as outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, our Board of Directors exercised reasonable judgment and considered numerous objective and subjective factors to determine the best estimate of the fair value of our Common Stock including:
• Lack of marketability of our Common Stock; price of our Common Stock sold to outside investors in arm’s length transactions, if any;
• Actual operating and financial performance;
• Current business conditions and projections;
• Hiring of key personnel and the experience of our management;
• The history of the Company and the introduction of new products and services;
• Likelihood of achieving a liquidity event, such as an initial public offering or a merger or acquisition of our company given prevailing market conditions;
• Illiquidity of share-based awards involving securities in a private company;
• External market conditions affecting the life sciences and medical devises industry sectors.
Our Common Stock valuations approach as of different valuation dates is as follows:
Enterprise Value (“EV”) Determination
• December 31, 2019 — EV was computed based on weighted average value from the Cost Approach and Market Approach (considering the private equity financings in the comparable companies);
• December 31, 2020 — EV was computed based on weighted average value from the Income Approach (discounted cash flow (“DCF”) method) and Market Approach (considering the private equity financings in the comparable companies). Under the DCF approach, the future value of the cash flow streams is discounted using cost of capital i.e. determined based on the stage of development of the Company;
• March 31 and June 30, 2021 — EV was computed was primarily based on the DCF method.
The equity value is then calculated by adjusting the cash (added) and debt (deducted) as of the valuation date and allocated to equity holders using a simple waterfall as we do not have a complex capital structure. A discount for lack of marketability (“DLOM”) of the Common Stock is then applied to arrive at an indication of value for the Common Stock on a minority and non-marketable basis.
If awards were granted a short period of time preceding the date of a valuation report where there was a material increase in valuation, we assessed the fair value used for financial reporting purposes after considering the fair value reflected in the subsequent valuation report and other facts and circumstances on the date of grant as discussed below. In such instances, the fair value we used for financial reporting purposes generally exceeded the exercise price for those awards.
In 2021 we signed an underwriter, introduced a second product with a shorter commercialization timeline and adjusted the current year costs to achieve FDA clearance and thereby impacted the DCF enterprise value. As a result, our equity value increased and our DLOM decreased. Using the benefit of hindsight, we determined that the straight-line calculation would provide the most reasonable conclusion for the valuation of our Common Stock on this interim date between valuations. The impact of this change increased stock-based compensation by $24,000 in the six months ended June 30, 2021.
For the September 12, 2021 grant, the factors our board of directors considered in determining the estimated fair value of our common stock in connection with the grant of these stock options included the results of our operations for the most recent quarter and the EV from the most recent contemporaneous valuation prepared by a third-party valuation firm.
42
In this prospectus, our board of directors received our first indication of the estimated preliminary price range for our Common Stock in this offering from the underwriter with a range of $5.50 to $6.50. This offering price assumes that the initial public offering has occurred and a public market for our common stock has been created. Therefore, the offering price excludes any marketability or illiquidity discount for our common stock, which was appropriately considered in our board of directors’ fair value determinations and can account for much of the difference between the offering price and the values determined by the board. Using the benefit of hindsight, and considering the DLOM, we determined that the straight-line calculation between the grant date and the estimated date that initial public offering may occur would provide the most reasonable conclusion for the valuation of our Common Stock on this interim date.
Options Granted:
The following table summarizes by grant date the number of shares subject to options granted from January 1, 2020, through the date of this Prospectus, the per share exercise price of the options and the estimated fair value per share of the options.
|
Grant Date |
Number |
Exercise Price |
Fair Value |
|||||
|
13-May-20 |
54,545 |
$ |
0.28 |
$ |
0.28 |
|||
|
14-Aug-20 |
37,090 |
$ |
0.28 |
$ |
0.28 |
|||
|
4-Feb-21 |
18,181 |
$ |
0.33 |
$ |
1.18 |
|||
|
22-Mar-21 |
23,636 |
$ |
0.33 |
$ |
2.28 |
|||
|
14-Jun-21 |
241,818 |
$ |
2.50 |
$ |
2.75 |
|||
|
12-Sep-21 |
72,727 |
$ |
2.81 |
$ |
3.83 |
|||
The aggregate intrinsic value of outstanding and vested stock options at June 30, 2021, was approximately $1,367,000 and $648,000 respectively. Based on the assumed initial offering price of $6.00 per share, the aggregate intrinsic value of options outstanding as of June 30, 2021 was $3,887,817 of which $1,447,830 related to vested options.
Equity transactions, including the accounting and classification of Common Stock and warrants, as well as accounting for modification to equity instruments, including but not limited to stock options.
Debt transactions, including the accounting and classification of convertible debt, as well as associate considerations and disclosures with any debt modifications.
Research and Development expenses are charged to expense as incurred and include, but not limited to, employee related expenses, fees associated with consultants supporting our research and development endeavors, expenses incurred under agreements with contract research organizations and costs associated with pre-clinical activities.
Cash flow and going concern assessment, in accordance with ASU 2014-15, requires us to evaluate the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued.
Related party transactions involve identifying, accounting for, and disclosing relationships and transactions with related parties.
43
Overview
Company Overview
We are a medical technology company primarily focusing on telemedicine solutions that enable the detection and monitoring of cardiac disease outside a healthcare facility setting. Our aim is to deliver innovative, remote diagnostic and monitoring technologies that can be used for patients anywhere, with initial offerings for ambulatory and emergency room use. We believe our products and services will benefit many stakeholders, including patients, healthcare providers, and healthcare payors. Our initial focus is providing diagnostic data to help physicians with care management of patients with cardiovascular disease. There are two major markets for our initial products: remote patient monitoring and the hospital Emergency Room (ER). First, we are developing our telehealth Product to address the rapidly growing field of remote patient monitoring. Our telehealth Product is comprised of a credit card sized, reusable ECG machine and a powerful cloud-based diagnostic software expert system. We believe that we are uniquely positioned to play a central role in remote monitoring of high-risk coronary artery disease patients, because of our highly accurate ischemia detection system, unlike other ambulatory cardiac monitors currently on the market which focus on arrhythmia detection. Secondly, we are applying the EKG interpretation engine of our platform technology to create a software tool for detecting heart attacks in the ER environment. This software tool is designed to enable emergency physicians to more accurately and quickly diagnose heart attacks than currently available tools.
To date, we have developed working prototypes for both our telehealth Product and our ER Product. The ER Product is currently undergoing additional engineering work that we believe will make it ready for the 510(k) clearance submission. Both Products were validated in three medical studies, designed by Harvard Medical School faculty. A series of scientific, peer reviewed publications describing the results of the studies and validation results are in preparation. One peer reviewed medical publication submission is planned for November 2021 and another for January 2022. These two publications will describe results of our two key studies: HIDES and B Score. In early 2022 we plan to publish results of a study on performance of our ER Product in the real-life environment of an ER. In July 2021 we submitted a technology abstract for presentation at the IEEE EMBS 2021 Conference; it was accepted. The abstract describes the technology foundation of our ER Product.
HeartBeam has three issued U.S. patents, seven pending U.S. utility patent applications (two are provisional patent applications and five are utility applications). Two of the pending utility applications have published, all of the remaining five pending cases are unpublished.
Market Overview
Chronic diseases are the number one burden on the healthcare system, driving up costs each year and cardiovascular illnesses are one of the top contributors. Regulators, payors and providers are focused on shifting the diagnosis and management of these conditions to drive better outcomes at lower cost. Connected medical solutions are expanding rapidly and are projected to reach $155 billion by 2026 at a compound annual growth rate (CAGR) of 17%. These solutions are socio-technical models for healthcare management and delivery using technology to provide healthcare services remotely and aims to maximize healthcare resources and provide increased, flexible opportunities for consumers to engage with clinicians and better self-manage their care, using readily available consumer technologies to deliver patient care outside of the hospital or doctor's office. The types of companies that make up this market include Accenture, IBM, SAP, GE Healthcare, Oracle, Microsoft, Airstrip Technology, Medtronic, Allscripts, Boston Scientific, Athenahealth, Cerner, Philips, Agamatrix, Qualcomm, and AliveCor.
The market for remote patient monitoring (RPM), one of the key areas of focus for chronic cardiovascular patients, is projected to reach $31.3 billion by 2023. In 2019, 1,800 hospitals in the US were using mobile applications to improve risk management and quality of care. The number in 2020 is likely larger as the onset of the COVID-19 pandemic greatly accelerated use and acceptance of telehealth by both patients and healthcare providers.
Cardiovascular disease is the number one cost to the healthcare system and is estimated to be responsible for 1 in every 6 healthcare dollars spent in the US. As cardiovascular disease is the leading cause of death worldwide, early detection, diagnosis, and management of chronic cardiac conditions are necessary to relieve the increasing burden on the healthcare infrastructure. Diagnostic tests such as ECGs are used to detect, diagnose and track numerous
44
cardiovascular conditions. We believe that the trend of moving diagnostic data collection and care for heart disease to the ambulatory setting is a permanent change. This trend will continue to place increased value on cost-effective diagnostic and monitoring technologies in the ECG market.
Time to intervention is one the key factors that influence medical outcome for a patient that is experiencing a heart attack. Yet, for a number of reasons, on average, a heart attack patient will wait about three hours before seeking help. That has very negative consequences on mortality rate for these patients as well as a market increase in the heart failure incidence in these patients. The huge increase in healthcare expenditures associated with that delay in seeking medical help is responsible for relative increase in heart failure rate of 8.7% for every 30 minutes of delay. Heart failure is one of the most expensive diagnoses to treat.
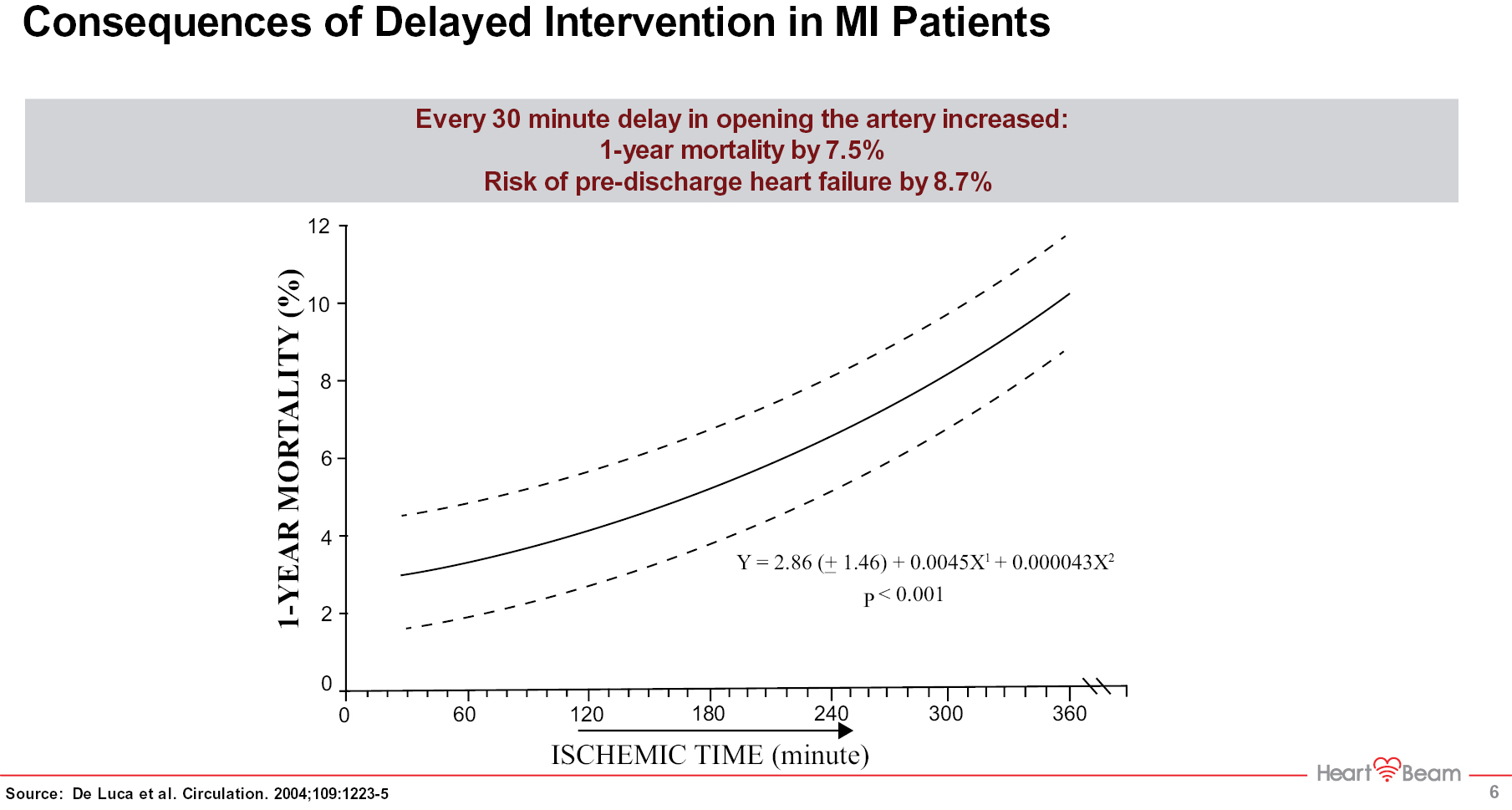
Our initial telemedicine Product will address the heart attack detection market as well as the market to monitor high-risk coronary artery disease (CAD) patients. CAD patients are at high risk for a heart attack. Currently there are no products on the market that are user friendly, easy to carry, and always with the patient in order to provide physicians and patients with timely and highly accurate information about potential Acute Coronary Syndrome (ACS) and Myocardial Infarction (MI) events. A tool that is always with the patient, that decreases time to intervention, and that decreases the number of unnecessary ER visits by chest pain patients would have a significant effect on saving lives, reducing incidence of heart failure and saving healthcare dollars. We believe our technology will address this problem and will provide a convenient, cost-effective, integrated telehealth solution, including software and hardware for physicians and their patients. There are approximately 18 million people in the US with coronary artery disease (CAD) who are considered at high risk for a heart attack, including 8 million who already have had prior intervention for a MI and are therefore considered to be at extreme risk.
In the US, mobile cardiac tests are primarily conducted through outsourced Independent Diagnostic Testing Facilities (IDTFs) or as part of Remote Patient Monitoring (RPM) system. Reimbursement rates vary, depending on the use case and generally are based on the value a technology offers to patients and healthcare providers. Actual reimbursed pricing is set by the Centers for Medicare & Medicaid Services (CMS), a part of the U.S. Department of Health and Human Services. Reimbursement rates for private insurers typically provide for similar or better reimbursement rates when compared to those set by the Government for Medicare and Medicaid.
In the ER environment, early and accurate diagnosis of a chest pain patient who is potentially having a heart attack is of immense importance. Guidelines state that every chest pain patient in an ER must receive an ECG within 10 minutes of presentation. Published literature evaluating physician accuracy in interpreting potential ST-segment elevation for myocardial infarction showed an overall accuracy of interpretation among true STEMIs was 66%. The need for increased ECG accuracy in detecting a heart attack in the ER is well defined, and an improved solution could result in saved lives and healthcare dollars. We have developed a prototype tool that, according to our initial study, offers a marked increase
45
in the accuracy of heart attack detection in ERs. We are currently developing, in partnership with Ximedica, a version of our ER Product to meet all FDA 510(k) clearance requirements. There are approximately 5,000 ER departments in the US and 137 million ER visits per year.
Products and Technology
The foundation of our novel technology is the concept of vectorcardiography (VCG), a technology that has long been seen as superior to ECGs in detecting MIs, but is no longer used clinically because of the difficulty experienced by physicians interpreting the output. We solved the crucial problem of recording three orthogonal (x, y and z) projections of the heart vector with a device that is sized like a credit card. The thickness of our credit card sized ECG signal collection device is approximately 1/8 inch (3mm) and weighs approximately 1 ounce (28 grams).
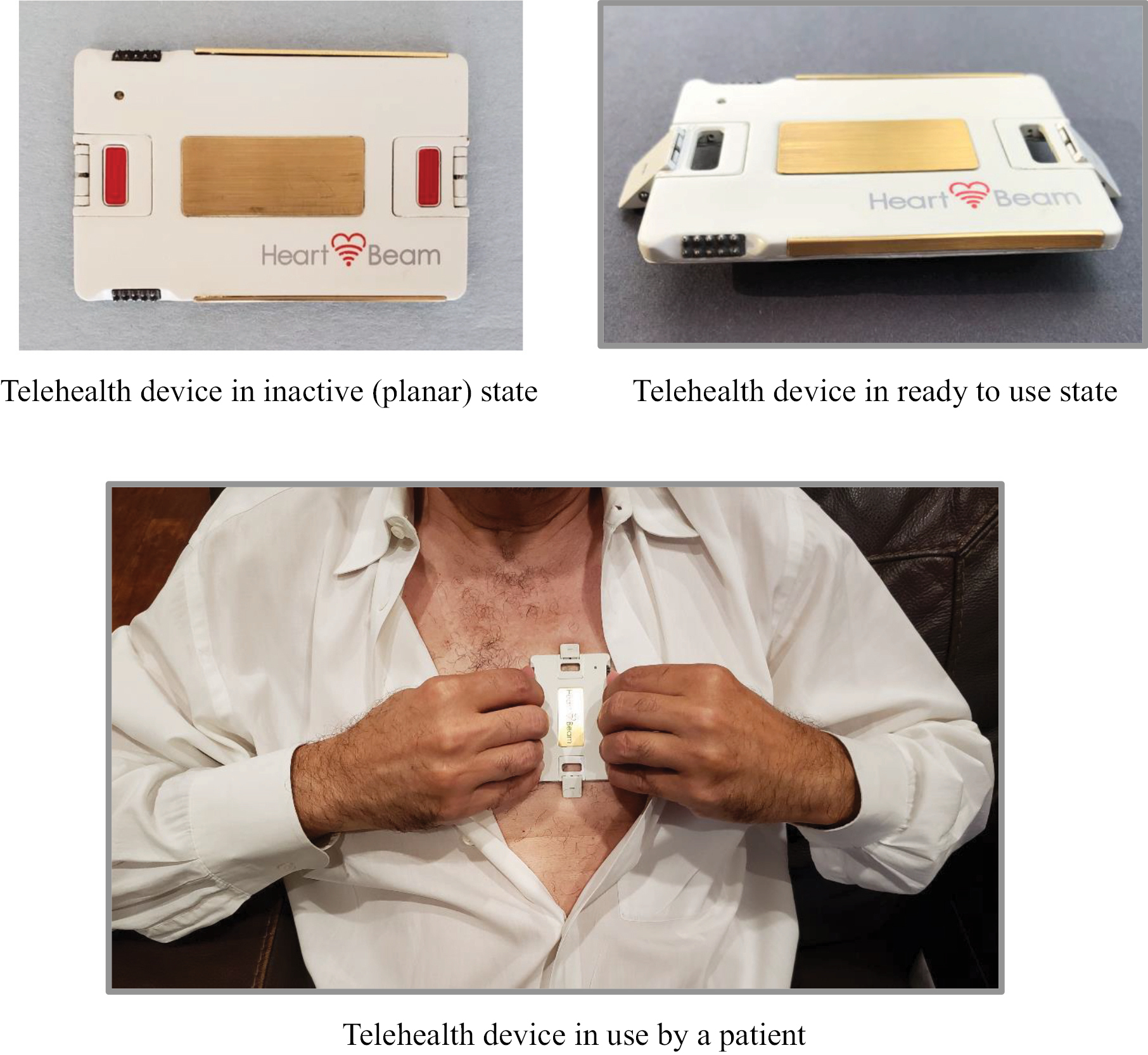
The core technology consists of a series of patented inventions and associated algorithms. In addition to the concept of using the VCG to get a more complete 3D characterization of cardiac activity, we use the concept of a baseline. Our MI marker is a differential marker that measures the change in cardiac parameters between an asymptomatic (baseline) recording and the symptomatic recording. It is personalized for every patient as every patient has a unique baseline. Our diagnostic performance gains in detecting MIs, when compared to a panel of cardiologists, are attributed to a more information rich cardiac information set offered by VCG and the fact that our MI market compares the baseline and symptomatic recordings.
This novel technology has resulted in two key products to date: a telehealth Product for high-risk cardiovascular patients that is comprised of a credit card-sized device and a powerful cloud-based diagnostic expert system and an MI detection system for ERs.
46
Our telehealth ECG collection device is the size of a credit card and has integrated electrodes. It does not rely on wires or self-adhesive electrodes to take a recording of cardiac signals. Unlike a standard 12-lead ECG machine that records signals in predetermined spots on a human body that were empirically determined, our approach is focused on recording three projections of the heart vector. The successful recording of the projections of the heart vector enables the synthesis of a 12-lead signal set as well diagnostic work in the space of 3D heart vectors.
There are obvious ease of use advantages when comparing our handheld device which can be carried inside the patient’s wallet and instantly self-applied vs. the current 12-lead ECG machine, a bulky piece of equipment with 10 wires and electrodes needing to be attached to patient’s body and requiring a trained professional to apply. In addition, there are diagnostic performance advantages, including, based on our initial study, increased accuracy in diagnosing MIs. The system is used by patients at home or elsewhere, with interpretation from their physicians, to assess whether their chest pain is truly the result of an MI.
Our telehealth system will be a prescription-only mobile health system intended for individuals with known or suspected heart disease. It helps guide physicians in choosing the best course of action for their patients who experience chest pain outside of a medical facility. Our system brings a medical grade ECG to patients and will enable them to receive a plan of action from a physician in a timely manner. For regular and scheduled monitoring of heart conditions, patients record a baseline 30 second cardiac reading using our device. When a patient experiences symptoms, such as irregular heartbeats or chest pain, the patient can simply open the smartphone app and press the credit card sized device against the chest to collect signals that can be converted to a 12-lead ECG. This derived 12-lead ECG is sent to the physician overlayed over a patient’s baseline ECG recording. In addition, the patient provides input on their symptoms that are sent, along with the ECG data, to the cloud for interpretation by a physician. A cloud-based algorithm processes the signals and displays the symptom description and patient history to a physician to analyze and make a plan of action. From start to finish, the process takes just a few minutes.
The telehealth system consists of:
1. A credit card sized cardiac electrical signal collection device. The device captures cardiac signals that represent x, y and z projections of the heart and transmits them via Bluetooth connection to a smartphone. The signal collection device is always with the patient as it easily fits in a wallet. It is easy to use as all that is required of the patient is that the device be pressed against the chest.
2. A user smartphone application that receives the cardiac signals from the HeartBeam signal collection device. The app has several functions: guiding the patient through the signal collection, asking about symptoms, displaying status of the data collection, and notifying the patient of the plan of action as determined by a physician. In addition, the app will contain Health Insurance Portability and Accountability Act (HIPAA) compliant video conferencing or text capabilities for the healthcare provider to communicate directly with the patient.
3. A cloud-based software system that serves three basic functions: (1) Performing a final check of the ECG signal quality, (2) Synthesizing a 12-lead ECG from the measured (recorded) 3 vector leads, and (3) Preparing a summary report for the physician. In order to facilitate a more accurate physician interpretation of the data, the software overlays the patient’s synthesized baseline 12 lead ECG waveform on the synthesized 12 lead ECG waveform from the current event. To ensure high signal quality, the system checks for noise levels in the recorded signals. Those signals that can be effectively filtered are accepted and those that have a noise level above an empirically established threshold are rejected. If a recorded signal is rejected, the user is asked to repeat the recording.
4. A web-based physician portal, which displays all of the relevant information for the physician to analyze: patient history, symptoms, baseline and current readings, synthesized 12 lead ECG, and recorded 3 vector leads. The HeartBeam physician portal assists physicians with their diagnostic interpretation by providing both the baseline 12-lead synthesized ECG and the 12-lead synthesized ECG that is under evaluation.
The market release of our telehealth Product will be in two generations. The Generation 1 product will have a limited feature set and will offer to the physician a pair of baseline and symptomatic ECGs and a symptoms report. This product is an excellent match for existing CPT remote patient monitoring reimbursement codes. The Generation 2 product will feature our proprietary MI marker as well as our diagnostic suggestion in addition to all features of Generation 1 product. Since Generation 2 will offer much increased medical value, we will seek a unique reimbursement code for this product.
47
The same core technology is used in the ER Product. In this application, the increased accuracy of detecting MIs by the ECG is of utmost importance. An ECG is the first diagnostic test a chest pain patient receives in the ER and it has a major impact on the patient’s subsequent clinical path. The ER Product has no hardware and introduces minimal change to the standard of care chest pain diagnostic path. It uses a baseline standard 12-lead ECG from the Electronic Medical Record (EMR) and the chest pain ECG that is being evaluated. It converts both of them to a VCG representation and utilizes our proprietary differential 3D VCG ischemia marker. While our 3D VCG ischemia marker is calculated in the 3D vector space the result is displayed as a simple diagnostic message, a diagnostic suggestion for the benefit of the ER physician. The physician never sees the VCG as it is only used inside our algorithm to arrive at a diagnostic suggestion for the physician, such as “Myocardial Infarction likely”. An initial clinical study indicates that the ER software offers considerable improvement in the accuracy of MI detection compared to a panel of experienced cardiologists interpreting a standard 12 lead ECG. The importance of increased accuracy of the first ECG interpretation for a chest pain patient presenting to an ER is great, potentially leading to faster intervention or avoiding unnecessary activation of the cardiac catheterization lab.
FDA Regulatory Path
We have defined the FDA 510(k) clearance paths for both Products and have contracted with two regulatory consultants to help us clear both products with the FDA.
Two predicate devices were identified that combined, we believe, will be acceptable to the FDA for our telehealth Generation 1 Product submission. The submission is planned to contain results of a validation study. The nature of this study will be a comparison of our synthesized 12-lead ECG recordings to standard 12-lead ECG recordings and to the synthesized ECG recordings from one of the predicate devices. We plan to submit our Generation 1 telehealth product to the FDA for a 510(k) clearance in the fourth quarter of 2022.
The predicate device for the ER Product was identified. It is widely used as it is part of a software package that one of the leading ECG machine manufacturers offers. The predicate device software makes a diagnostic suggestion in regard to a potential MI diagnosis. Our ER software Product will also make a diagnostic suggestion to the ER physician. In our HIDES study we have shown superiority in detecting ischemia (ACS) over a panel of cardiologists. The study that will be used for the FDA 510(k) clearance application will be a retrospective study of patient's electronic medical records that contain the patient's chest pain ECGs at the presentation to the ER and their baseline asymptomatic ECG recordings. The diagnostic suggestion of the predicate device software, which is already contained in the EMR, will be compared to the diagnostic suggestion of our Product. We have obtained Independent Review Board (IRB) approval for this study at the University of Tennessee. We plan to submit our ER Product for FDA 510(k) clearance in the second quarter of 2022.
Market Opportunity
ECGs are a key diagnostic test utilized in the diagnosis and monitoring of cardiovascular disease, the number one cause of death worldwide. In the US in 2016, there were 121.5 million adults living with cardiovascular disease and 18.3 million adults with diagnosed coronary artery disease. The market size is increasing, due to an aging population and lifestyle choices.
Every 40 seconds someone in the US has a heart attack, or myocardial infarction (MI). Unfortunately, there is no way for patients to tell whether the symptoms they are experiencing are due to an MI, or some other more benign condition such as indigestion. As a result, patients often ignore symptoms and delay seeking care, which leads to worse outcomes and increased mortality. On the other hand, many patients who go to the Emergency Room with chest pain are not experiencing an MI. Chest pain is the second most common reason for an ER visit, yet fewer than 20% of chest pain ER visits result in a diagnosis of a life-threatening condition. These unnecessary ER visits lead to well over $10 billion in unnecessary healthcare expenditures.
Most ECGs are conducted in a healthcare facility setting using a 12-lead ECG machine, the gold standard. ECGs taken outside of healthcare facilities are expected to grow more quickly than in-hospital ECGs. Monitoring cardiac patients outside of a hospital is a growing trend, as it is less expensive and provides a better patient experience. However, while ambulatory cardiac monitoring devices are often much easier for patients to use, they have fewer leads than the gold standard and cannot offer as comprehensive a picture of cardiac health as the gold standard.
48
While a standard 12-lead ECG readout is of great medical value, it is simply impractical to have a machine next to patients when they experience symptoms outside the clinical setting, since recording the event requires attaching multiple electrodes to the patient’s body with professional assistance. While existing technologies use single lead patches or similar approaches to monitor arrhythmias, these technologies do not provide information to the physician on the potential of the life-threatening conditions of ACS or heart attacks.
Our telehealth technology addresses these market needs and has several key attributes that make it a good fit for these patients. Our telehealth Product is designed to be used when symptoms occur and potentially for lifelong patient usage. The device fits in a wallet and is always near patient and ready to be used for recording a cardiac event. It enables real-time cardiac data transmission during a telemedicine visit. It offers a recorded 3 vector leads set of signals and a 12-lead derived ECG set of signals. Physicians will prescribe our solution to chronic cardiovascular patients for period of months to years, thereby enabling prolonged data collection and delivering a more complete picture for diagnosis. This will also enable use of artificial intelligence on our future database that will have a unique set of longitudinal 12-lead ECGs.
Market Strategy
Our goal is to establish our products as key solutions for cardiology practices and hospitals. Our efforts to enter the market involve establishing clinical evidence and demonstrating the cost-effectiveness of adopting our products. For both the telehealth and the ER Products, the initial geographic market is the United States.
We believe that both the telehealth and ER Products will be subject to the US FDA’s 510(k) review process. We are in the process of preparing regulatory submissions for both Products.
For the telehealth Product, the primary customers are cardiology practices and the cardiology departments of hospitals. Healthcare insurers are another important customer, as they will benefit from the reduced costs to the healthcare system. We are working to develop new clinical studies and publish results of completed clinical studies that will demonstrate real world cost-effectiveness of the use of the solution.
A key element of our strategy is obtaining reimbursement for the telehealth Product. This strategy has two stages. In the short term, we expect that physicians will use existing Remote Patient Monitoring (RPM) reimbursement codes. We believe that the telehealth Product is a compelling offering among RPM technologies, as it is uniquely positioned to assess ACS and heart attacks among high-risk cardiac patients. Because our telehealth solution offers ACS and, in general, CAD detection capability that single lead ECG technologies do not offer, we are uniquely positioned to provide remote monitoring of high-risk CAD patients. In the longer term, we will conduct additional clinical trials that demonstrate the clinical efficacy and cost effectiveness of our solution and will work to secure reimbursement specific to the telehealth solution.
RPM codes provide practices with payment for providing covered services. The main RPM codes are:
• CPT 99453: Remote monitoring of physiologic parameter(s), initial; set-up and patient education on use of equipment
• CPT 99454: Remote monitoring of physiologic parameter(s), initial; device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 days
• CPT 99457: Remote physiologic monitoring treatment management services, clinical staff/physician/ other qualified health care professional time in a calendar month requiring interactive communication with the patient/caregiver during the month; initial 20 minutes
• CPT 99458: Remote physiologic monitoring treatment management services, clinical staff/physician/ other qualified health care professional time in a calendar month requiring interactive communication with the patient/caregiver during the month; additional 20 minutes
CPT 99453 is paid one-time per patient, with the average CMS payment rate of $21. The technical code CPT 99454 and the professional code CPT 99457 are paid monthly, with a combined average CMS payment rate of $119. Private payers will pay at different amounts.
Practices will bill payers for services related to the core HeartBeam telehealth Product, potentially bundled with a third-party blood pressure measurement device, on a monthly payment. Under this model, the company will negotiate with payers for a per patient per month fee for the ongoing HeartBeam telehealth service, which also will include an amortized charge for the cost of the device.
49
We will explore other business models. For example, hospitals face CMS penalties if their 30-day readmission rates for patients who are discharged after an MI exceed certain thresholds. These CMS penalties are levied on all hospital CMS payments, so the impact can be significant. Our telehealth Product can be a tool to help hospitals manage these patients during the first 30 days. We will explore models in which hospitals pay for the device and for the initial 30 days of service. In addition, we will explore models for value-based care, in which the use of the telehealth Product reduces overall costs.
We expect to develop a direct sales force and to target large hospitals and integrated practices. These are sophisticated customers, and we will use technical presentations, peer reviewed clinical data, and demonstration projects to achieve penetration of this market. We will continue to expand our medical advisory board, will conduct clinical trials with leading cardiologists to generate the necessary evidence, and will establish reference sites among these customers.
Our long-term strategy is to generate sufficient clinical and cost-effectiveness evidence to generate reimbursement coverage and payment specifically for the HeartBeam telehealth solution. We expect to be able to demonstrate significant clinical benefits for patients and savings to the health care system, justifying reimbursement levels well in excess of the amount paid through the RPM pathway. This is expected to greatly expand the Product’s market potential.
Our primary marketing strategy for the telehealth Product is the generation of clinical and cost-effectiveness data and the establishment of reference sites to appeal to the target cardiology customers. Over time, we expect to help our customers educate potential patients about the benefits and the use of the HeartBeam solution and we will create patient-facing educational materials to assist our customers.
For the ER Product, the primary customers are hospital Emergency Rooms. As with the telehealth Product, we will publish clinical studies on the effectiveness of the Product. In addition, we will develop financial models demonstrating the cost-effectiveness of the approach and will establish reference sites who are using the Product. We do not expect to obtain specific reimbursement for the ER Product but intend to demonstrate that the purchase of the software would result in clinical and economic benefits to the institution.
We will establish a direct sales network with relationships and experience selling to ERs. In addition, we will identify leading Emergency Physicians to conduct clinical studies and generate real-world experience with the Product.
Clinical Data
HeartBeam has performed three clinical studies to assess performance of our technologies.
1. HIDES — (HeartBeam Ischemia Detection Study) included 66 patients whose coronary arteries were occluded during a Percutaneous Coronary Intervention (PCI) and had electrical signals simultaneously collected by both a traditional 12-lead ECG and our vector signal-based device.
ECG recordings:
66 baseline recordings for each patient were taken during patient enrolment and prior to the balloon inflation. These were ischemia negative recordings.
120 recordings were taken during balloon occlusions in various arteries. These were ischemia positive recordings. There were a total of 186 diagnostic recordings: 66 ischemia negative recordings and 120 ischemia positive recordings.
Study design:
The HeartBeam automated ischemia marker is based on the vector difference between ST vectors of the symptomatic and baseline recordings. Human reading results were obtained by averaging results from three expert readers (two electrophysiologists and one invasive cardiologist) who were presented with the 186 standard 12-lead ECG recordings. These readings were performed in two sessions four weeks apart. A total of six readings were averaged to arrive at human readers’ ischemia detection performance.
50
Results:
The automated HeartBeam ischemia marker was superior to human expert reading for detecting acute ischemia (66 patients, 120 balloon occlusions; 186 total recordings) using ECG signals only:
|
SENSITIVITY |
SPECIFICITY |
ACCURACY |
||||
|
Human readings |
71.94 |
70.96 |
71.59 |
|||
|
HeartBeam ischemia marker |
91.70 |
95.50 |
93.00 |
|||
|
p<0.01 |
p<0.01 |
p<0.01 |
The ischemia marker showed an area under the curve (AUC) in the receiver operating characteristic (ROC) curve (ROC) of 93.6%. HeartBeam’s marker accuracy was consistent all across the three culprit arteries (LAD, LCX, RCA), p=1.00. There was no statistically significant difference in accuracy between three human readers (p=1.00).
Synthetized vs, standard 12 lead ECG:
The synthetized 12 lead ECG was obtained using an individual transformation matrix obtained from standard 12 lead signals. Comparison of the standard 12 lead and synthetized 12 lead ECGs were performed with standard correlation analyses. Results for Pearson’s correlation coefficient R between standard 12 lead and synthetized 12 lead ECGs were:
P wave: R = 0.937;
QRS complex: R = 0.994;
T wave: R = 0.975
They are shown in the graphical form for all 186 pairs of signals (standard and synthesized 12-lead):
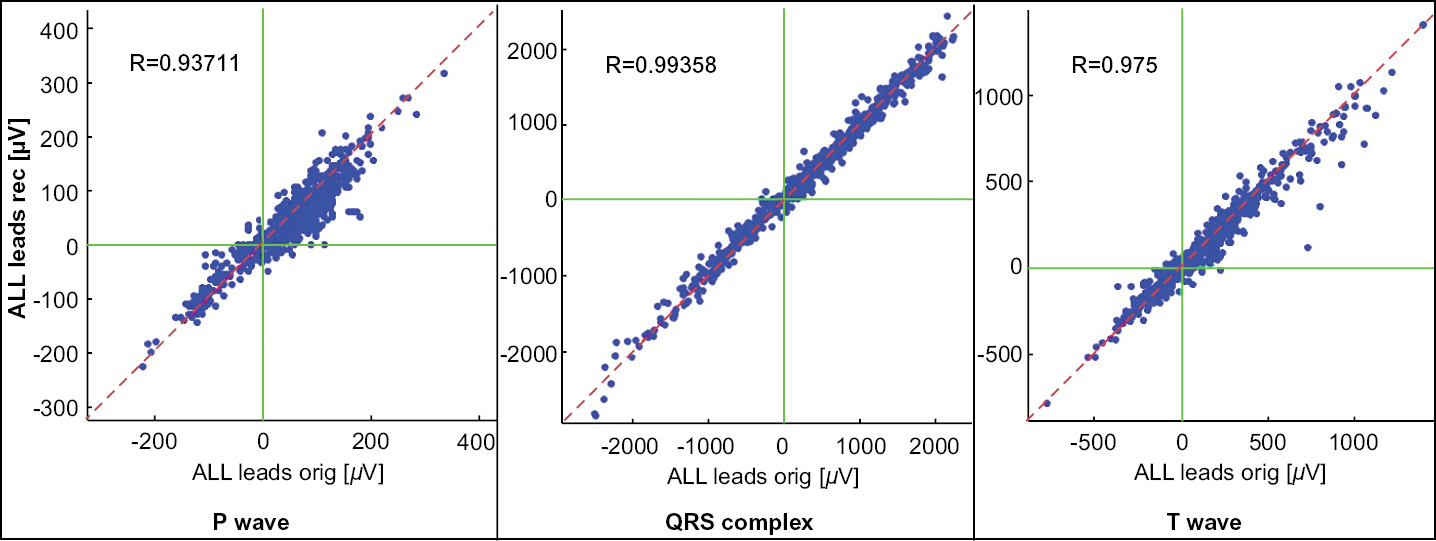
Pearson’s correlation coefficient is the test statistic that measures the statistical relationship between two continuous variables A value of 1 implies that a linear equation describes the relationship perfectly; value of 0 implies that there is no linear correlation between the variables
2. B Score — Evaluation of sensitivity and specificity of the HeartBeam diagnostic algorithm in diagnosing patients with ACS in the Emergency Room setting.
Study design and enrolment:
Enrolled were all patients presenting to an ER with chest pain or other symptoms suggestive of ACS who a) answered questions about risk factors and chest pain characteristics and b) had standard 12-lead ECG and HeartBeam ECG recorded 3 times with 10-15 minutes intervals between recordings. The final decision whether a patient was having ACS was determined by 3 cardiology experts (gold standard panel) based on discharge diagnosis and one week follow up data.
An additional HeartBeam ECG recording was taken between 9 and 12 months after the initial visit, when ST resolution was completed in the majority of cases, and this recording was used as the baseline ECG in HeartBeam’s diagnostic algorithm.
The same set of data — risk factors, chest pain characteristics and three recordings — were both used for evaluation of the HeartBeam algorithm and presented to three expert cardiologists (evaluation panel) for a blinded evaluation.
51
Results:
110 ER patients presenting with chest pain of which 29 (26%) with ACS (per gold standard panel), underwent HeartBeam assessment as well as assessment by the evaluation cardiologist panel. Sensitivity of the HeartBeam algorithm was 97% (27/28) and specificity was 56% (45/81). The only positive patient missed by the algorithm had known coronary disease with typical anginal episode resulting in a troponin leak. The average sensitivity and specificity of the evaluation cardiologists panel was 93% and 49%, respectively. The diagnostic performance of the HeartBeam algorithm in determining presence of the ACS in these patients was statistically indifferent from the performance of the evaluation cardiologist panel (p>0.42).
This result indicates that the quality of the advice produced by our expert system will be extremely valuable to the physician who is assessing the condition of a patient in a telehealth environment.
3. ISPEC — evaluation of the ECG signal quality and specificity of the ACS detection (false positive rate) in real-life use of the HeartBeam (HB) device by non-symptomatic patients.
Study design and enrolment:
The study included 30 participants, healthy volunteers and patients with different cardiac disturbances. The participants recorded three HeartBeam recordings three times daily, for 3 to 7 days. The ECG signal quality was evaluated as the percentage of recordings rejected by the HeartBeam cloud-based software due to insufficient signal quality. The specificity of HeartBeam in classification of non-ischemic recordings was performed by application of HeartBeam’s ACS detection algorithm. It was assumed that none of the patients was ischemic during the study period, as none reported chest pain symptoms.
Study results:
The study generated a total of 1845 recordings; 15.2% (282/1845) of the recordings were rejected by HeartBeam due to insufficient signal quality and had to be repeated. The false positive rate of the HeartBeam algorithm in classification of non-ischemic recordings was zero. In other words, the specificity was 100% in real-life use of our system in asymptomatic patients.
Competition
The cardiac monitoring and detection market is characterized by rapid technological change and strong competition. There are numerous companies developing technologies that are competitive, in a broad sense, to our products, and many of these companies have significantly greater resources than we do.
In the category of ambulatory (telehealth) cardiac monitors — devices that are intended to be used outside of a health facility setting — there are two major segments: consumer devices and devices prescribed for ACS.
Consumer devices
The consumer device segment consists of devices that are FDA cleared but are sold directly to patients, without a prescription. Generally, these devices are single lead ECG devices intended to recognize heart rhythm abnormalities, such as atrial fibrillation, but are not intended for ischemia detection or for life threatening conditions such as heart attack.
• Apple Inc, a public company located in Cupertino, CA, produces the Apple Watch, which includes ECG functionality. The Apple Watch is a single lead ECG with two electrodes that contact the wrist and the finger and is intended to detect only Atrial Fibrillation.
• AliveCor Inc, a private company located in Mountain View, CA, produces the KardiaMobile and KardiaMobile 6L devices. These devices are intended to detect heart rhythm irregularities, such as Atrial Fibrillation, only.
• Google Inc, a public company located in Mountain View, CA, produces the Fitbit Sense smartwatch and ECG app. The Fitbit Sense watch is a single lead ECG with two electrodes that contact the fingers and is intended to detect only Atrial Fibrillation.
• Samsung Electronics Co., Ltd, based in Seoul, South Korea, is publicly traded in Korea. It produces the Galaxy Watch3 and Galaxy Watch Active2 smartwatches with ECG functionality, intended to detect only Atrial Fibrillation.
52
Devices prescribed for ischemia detection
There are a small number of devices that have been cleared by FDA to be used outside of healthcare facilities that provide information for patients with potential ischemic events such as MIs.
• Angel Medical Systems, Inc. is a private company based in Eatontown, NJ. The AngelMed Guardian is an implantable cardiac monitor for patients who are deemed to be extremely high risk for an MI. Physicians implant the AngelMed Guardian in patients. The company believes that the HeartBeam device will be a viable alternative to the AngelMed Guardian, as it does not require an implant and does not have a high up-front cost.
• SHL Telemedicine Ltd., is based in Tel Aviv, Israel and is publicly traded. It produces Smartheart Pro, a 12 lead ECG indicated for patient use at home. Smartheart Pro is larger and more complex than our telehealth solution, requiring the placement of an electrode belt, two underarm electrodes and a waist electrode, and moistening the areas before use. Most patients would find this technology not suited to be carried with them at all times because of the large size and complex lead attachment procedure.
There are several competitors in the category of software that automatically analyzes 12 lead ECGs performed in healthcare facilities, specifically in an ER. Major competitors in this market include the following:
• General Electric, a publicly traded company based in Chicago, IL produces a line of ECG equipment. The company also has developed the GE Marquette 12SL ECG analysis program, which analyzes the ST segment of the ECG to detect potential cardiac ischemia. It does not use the 3D vector approach in deriving a diagnostic suggestion.
• Koninklijke Philips N.V., a publicly traded company based in Amsterdam, NL, produces a range of ECG products, including products that feature the DXL algorithm for resting ECGs. The Philips DXL algorithm monitors the ST segment to detect STEMI. It does use the 3D vector approach in deriving a diagnostic suggestion.
Intellectual Property
Our innovations are protected with a strong patent portfolio. For a limited number of aspects of our proprietary technology we rely on trade secret protection. It is our view that the combination of these two methods of intellectual property protection maximizes our chances for success.
HeartBeam has three issued U.S. patents (U.S. 10,433,744, U.S. 10,117,592 and U.S. 11071490), seven pending U.S applications (pending patent applications and two pending provisional applications). Two of the pending applications have published, all of the remaining five pending cases are unpublished. Outside of the U.S., HeartBeam has two pending EU utility patent applications, one pending Chinese utility patent application and one pending PCT applications. The issued patents are predicted to expire between April 11, 2036 and November 18, 2039.
The issued and pending U.S. patent applications cover compact systems for remote detection and/or diagnosis of acute myocardial infarction (AMI). The pending EU and Chinese patent applications correspond to the pending and issued US cases. The pending PCT (Patent Cooperation Treaty) applications cover methods and apparatuses for automatic cardiac diagnosis as well as compact systems including retractable electrodes.
53
A table summarizing our IP portfolio is shown below:
|
Patent Type |
Application no. |
Status |
Predicted Expiration |
Title |
||||||
|
Utility |
US |
15/096,159 US 10,433,744 |
Issued |
Sep 15, 2036 |
MOBILE THREE-LEAD CARDIAC MONITORING DEVICE AND METHOD FOR AUTOMATED DIAGNOSTICS Methods and apparatuses for remote and detection and/or diagnosis of acute myocardial infarction (AMI). |
|||||
|
Utility |
US |
15/632,155 US 10,117,592 |
Issued |
Apr 11, 2036 |
MOBILE THREE-LEAD CARDIAC MONITORING DEVICE AND METHOD FOR AUTOMATED DIAGNOSTICS Methods and apparatuses for remote and detection and/or diagnosis of acute myocardial infarction (AMI). |
|||||
|
Utility |
US |
17/092,152 |
Published |
Apr 11, 2036 |
MOBILE THREE-LEAD CARDIAC MONITORING DEVICE AND METHOD FOR AUTOMATED DIAGNOSTICS Methods and apparatuses for remote and detection and/or diagnosis of acute myocardial infarction (AMI). |
|||||
|
Utility |
US |
17/202,299 US 11,071,490 |
Issued |
Apr 11, 2036 |
ELECTROCARDIOGRAM PATCH DEVICES AND METHODS Methods and apparatuses for remote and detection and/or diagnosis of acute myocardial infarction (AMI). |
|||||
|
Utility |
CN |
201680030550.5 |
Published |
Apr 11, 2036 |
MOBILE THREE-LEAD CARDIAC MONITORING DEVICE AND METHOD FOR AUTOMATED DIAGNOSTICS Methods and apparatuses for remote and detection and/or diagnosis of acute myocardial infarction (AMI). |
|||||
|
Utility |
EU |
16777474.4 |
Published |
Apr 11, 2036 |
MOBILE THREE-LEAD CARDIAC MONITORING DEVICE AND METHOD FOR AUTOMATED DIAGNOSTICS Methods and apparatuses for remote and detection and/or diagnosis of acute myocardial infarction (AMI). |
|||||
|
Utility |
EU |
19894815.0 |
Pending |
Nov 18, 2039 |
HAND HELD DEVICE FOR AUTOMATIC CARDIAC RISK AND DIAGNOSTIC ASSESSMENT Method and apparatus for performing automatic cardiac diagnosis. |
|||||
|
Utility |
US |
17/296,669 |
Pending |
Nov 18, 2039 |
HAND HELD DEVICE FOR AUTOMATIC CARDIAC RISK AND DIAGNOSTIC ASSESSMENT Method and apparatus for performing automatic cardiac diagnosis. |
|||||
|
Utility |
PCT |
PCT/US2020/032556 |
Published |
N/A |
COMPACT MOBILE THREE-LEAD CARDIAC MONITORING DEVICE Compact, mobile three-lead cardiac monitoring devices for remote detection and/or diagnosis of cardiac events. |
54
|
Patent Type |
Application no. |
Status |
Predicted Expiration |
Title |
||||||
|
Provisional |
US |
63/113,181 |
Pending |
N/A |
COMPACT MOBILE THREE-LEAD CARDIAC MONITORING DEVICE WITH HYBRID ELECTRODE Compact, mobile three-lead cardiac monitoring devices for remote detection and/or diagnosis of cardiac events. |
|||||
|
Provisional |
US |
63/133,669 |
Pending |
N/A |
AMBULATORY ELECTROCARDIOGRAM PATCH DEVICES AND METHODS Cardiac monitoring patch devices (e.g., an ECG patch for 12-lead detection) for remote detection and/or diagnosis of cardiac events (e.g., acute myocardial infarction). |
We have entered into, and generally plan to continue to enter into, non-disclosure, confidentiality and intellectual property assignment agreements with all new employees as a condition of employment. In addition, we intend to generally enter into confidentiality and non-disclosure agreements with consultants, manufacturers’ representatives, distributors, suppliers and others to attempt to limit access to, use and disclosure of our proprietary information. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
The ownership of all filed patents is assigned to HeartBeam, Inc. At this time three additional patent applications are being drafted by us.
Research and Development
The primary objective of our research and development program is to provide innovative, user friendly telehealth solutions with high medical value. To date, we have been highly successful in developing our initial cardiovascular Products. The emphasis has been on developing a user-friendly solution that is always with the patient and that provides assistance to physicians in diagnosing heart attacks in chest pain patients.
Our Research and Development team is largely based in Belgrade, Serbia. We have assembled a highly capable team currently consisting of seven PhD level contributors who are credited with developing our key inventions and patents. The diverse group includes two nuclear physicists, three signal processing specialists, and two biomedical engineers.
We plan to utilize this team in the future and expect that the key members of this team will transition to full time employees. Future research and development efforts will focus on the application of signal processing and artificial intelligence to address a range of cardiac conditions.
Future Products
Our core technology — the heart vector approach adopted and invented by our scientific team — is a platform technology that can provide diagnostic solutions to a variety of cardiovascular patients. Our plans call for expanding to solutions to diagnose all major cardiac conditions that are diagnosed by ECGs.
Our future plans include the development of a 12-lead capable patch ECG monitor that will provide advantages over existing single-lead ECG patch Products such as the Zio-XT from iRhythm Technologies, Inc. Our approach offers a 12-lead ECG with a patch that is very similar, in dimensions and look and feel, to the currently available single lead ECG patches. Providing standard of care 12-lead ECG capabilities will have significant diagnostic advantages over a single lead.
While our initial telehealth Product is powered by a software expert system that serves as a diagnostic aid to a physician, we will be working on developing an AI based diagnostic system that will supplement our diagnostic expert system.
55
Government Regulation
General
Our proposed products are subject to regulation by the U.S. Food and Drug Administration (FDA) and various other federal and state agencies, as well as by foreign governmental agencies. These agencies enforce laws and regulations that govern the development, testing, manufacturing, labeling, advertising, marketing and distribution, and market surveillance of our medical device products.
In addition to those indicated below, the only other regulations we encounter are regulations that are common to all businesses, such as employment legislation, implied warranty laws, and environmental, health and safety standards, to the extent applicable. We will also encounter in the future industry-specific government regulations that would govern our products, if and when developed for commercial use. It may become the case that other regulatory approvals will be required for the design and manufacture of our products and proposed products.
U.S. Regulation
The FDA governs the following activities that HeartBeam performs, will perform, upon the clearance or approval of its Product, or that are performed on its behalf, to ensure that medical products distributed domestically or exported internationally are safe and effective for their intended uses:
• product design, and development
• product safety, testing, labeling and storage
• record keeping procedures; and
• product marketing.
There are numerous FDA regulatory requirements governing the approval or clearance and subsequent commercial marketing of our products. These include:
• the timely submission of product listing and establishment registration information, along with associated establishment user fees;
• continued compliance with the Quality System Regulation, or QSR, which require specification developers and manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process;
• labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication;
• clearance or approval of product modifications that could significantly affect the safety or effectiveness of the device or that would constitute a major change in intended use;
• Medical Device Reporting regulations (MDR), which require that manufacturers keep detailed records of investigations or complaints against their devices and to report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur;
• adequate use of the Corrective and Preventive Actions process to identify and correct or prevent significant systemic failures of products or processes or in trends which suggest same;
• post-approval restrictions or conditions, including post-approval study commitments;
• post-market surveillance regulations, which apply when necessary, to protect the public health or to provide additional safety and effectiveness data for the device; and
• notices of correction or removal and recall regulations.
56
Depending on the classification of the device, before HeartBeam can commercially distribute medical devices in the United States, it has to obtain, either prior 510(k) clearance, 510(k) de-novo clearance or premarket approval (PMA), from the FDA unless a respective exemption applies to the device under review by the FDA.
The FDA classifies medical devices into one of three classes based on the degree of risk associated with each medical device and the extent of regulatory controls needed to ensure the device’s safety and effectiveness:
• Class I medical devices, which are low risk and subject to only general controls (e.g., registration and listing, medical device labeling compliance, MDRs, Quality System Regulations, and prohibitions against adulteration and misbranding) and, in some cases, to the 510(k) premarket clearance requirements;
• Class II medical devices, which are moderate risk and generally require 510(k) or 510(k) de-novo premarket clearance before they may be commercially marketed in the United States as well as general controls and potentially special controls like performance standards or specific labeling requirements; and
• Class III medical devices, which are devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device. Class III medical devices generally require the submission and approval of a PMA supported by clinical trial data.
The custom software and hardware of our products, we believe, are classified as Class II. Class II medical devices are those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish special controls. Special controls can include performance standards, post-market surveillance, patient histories and FDA guidance documents. Premarket review and clearance by the FDA for these devices is generally accomplished through the 510(k) or 510(k) de-novo premarket notification process. As part of the 510(k) or 510(k) de-novo notification process, the FDA may have required the following:
• Development of comprehensive product description and indications for use
• Completion of extensive preclinical tests and preclinical animal studies, performed in accordance with the FDA’s Good Laboratory Practice (GLP) regulations
• Comprehensive review of predicate devices and development of data supporting the new product’s substantial equivalence to one or more predicate devices
• If appropriate and required, certain types of clinical trials (IDE submission and approval may be required for conducting a clinical trial in the US).
Clinical trials involve use of the medical device on human subjects under the supervision of qualified investigators in accordance with current Good Clinical Practices (GCPs), including the requirement that all research subjects provide informed consent for their participation in the clinical study. A written protocol with predefined end points, an appropriate sample size and pre- determined patient inclusion and exclusion criteria, is required before initiating and conducting a clinical trial. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s Investigational Device Exemption, or IDE, regulations that among other things, govern investigational device labeling, prohibit promotion of the investigational device, and specify recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk,” as defined by the FDA, the agency requires the device sponsor to submit an IDE application, which must become effective prior to commencing human clinical trials. The IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA denies the application or notifies the company that the investigation is on hold and may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE that requires modification, the FDA may permit a clinical trial to proceed under a conditional approval. In addition, the study must be approved by, and conducted under the oversight of, an Institutional Review Board (IRB) for each clinical site. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but it must still follow abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements.
57
Given successful completion of all required testing, a detailed 510(k) premarket notification or 510(k) de-novo will be submitted to the FDA requesting clearance to market the product. This notification will include all relevant data from pertinent preclinical and clinical trials, together with detailed information relating to the product’s manufacturing controls and proposed labeling, and other relevant documentation.
A 510(k) clearance letter from the FDA authorizes commercial marketing of the device for one or more specific indications of use.
After 510(k) clearance, HeartBeam is required to comply with a number of post-clearance requirements, including, but not limited to, Medical Device Reporting and complaint handling, and, if applicable, reporting of corrective actions. Also, quality control and manufacturing procedures must continue to conform to QSRs. The FDA periodically inspects manufacturing facilities to assess compliance with FDA’s Quality System Regulations (QSRs), which impose extensive procedural, substantive, and record keeping requirements on medical device manufacturers. In addition, changes to the manufacturing process are strictly regulated, and, depending on the change, validation activities may need to be performed. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with QSRs and other types of regulatory controls.
After a device receives 510(k) clearance from the FDA, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use or technological characteristics, requires a new 510(k) clearance or could require a PMA. The FDA requires each manufacturer to make the determination of whether a modification requires a new 510(k) notification or PMA in the first instance, but the FDA can review any such decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance or PMA for a particular change, the FDA may retroactively require the manufacturer to seek 510(k) clearance or PMA. The FDA can also require the manufacturer to cease U.S. marketing and/or recall the modified device until additional 510(k) clearance or PMA approval is obtained.
The FDA and the Federal Trade Commission, or FTC, will also regulate the advertising claims of HeartBeam’s products to ensure that the claims it makes are consistent with its regulatory clearances, that there is scientific data to substantiate the claims and that product advertising is neither false nor misleading.
We will apply for 510(k) clearance for both our ER software only Product and the software and hardware components of our telehealth Product. To obtain 510(k) clearance, a company must submit a notification to the FDA demonstrating that its proposed device is substantially equivalent to a predicate device (i.e., a device that was in commercial distribution before May 28, 1976, a device that has been reclassified from Class III to Class I or Class II, or a 510(k)-cleared device). The FDA’s 510(k) clearance process generally takes from three to 12 months from the date the application is submitted but also can take significantly longer. If the FDA determines that the device or its intended use is not substantially equivalent to a predicate device, the device is automatically placed into Class III, requiring the submission of a PMA. Once the information is submitted, there is no guarantee that the FDA will grant a company 510(k) clearance for its pipeline products, and failure to obtain the necessary clearances for its products would adversely affect its ability to grow its business. Delays in receipt or failure to receive the necessary clearances, or the failure to comply with existing or future regulatory requirements, could reduce its business prospects.
Devices that cannot be cleared through the 510(k) process due to lack of a predicate device but would be considered low or moderate risk may be eligible for the 510(k) de-novo process. In 1997, the Food and Drug Administration Modernization Act, or FDAMA added the de novo classification pathway now codified in section 513(f)(2) of the FD&C Act. This law established an alternate pathway to classify new devices into Class I or II that had automatically been placed in Class III after receiving a Not Substantially Equivalent, or NSE, determination in response to a 510(k) submission. Through this regulatory process, a sponsor who receives an NSE determination may, within 30 days of receipt, request FDA to make a risk-based classification of the device through what is called a “de novo request.” In 2012, section 513(f)(2) of the FD&C Act was amended by section 607 of the Food and Drug Administration Safety and Innovation Act (FDASIA), in order to provide a second option for de novo classification. Under this second pathway, a sponsor who determines that there is no legally marketed device upon which to base a determination of substantial equivalence can submit a de novo request to FDA without first submitting a 510(k).
In the event that a company receives a Not Substantially Equivalent determination in response to a 510(k) submission, the device may still be eligible for the 510(k) de-novo classification process.
58
Devices that cannot be cleared through the 510(k) or 510(k) de-novo classification process require the submission of a PMA. The PMA process is much more time consuming and demanding than the 510(k) notification process. A PMA must be supported by extensive data, including but not limited to data obtained from preclinical and/or clinical studies and data relating to manufacturing and labeling, to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device. After a PMA application is submitted, the FDA’s in-depth review of the information generally takes between one and three years and may take significantly longer.
We also need to establish a suitable and effective quality management system, which establishes controlled processes for our product design, manufacturing, and distribution. We plan to do this in compliance with the internationally recognized standard ISO 13485:2013 Medical Devices — Quality Management Systems — Requirements for Regulatory Purposes. Following the introduction of a product, the FDA and foreign agencies engage in periodic reviews of our quality systems, as well as product performance and advertising and promotional materials. These regulatory controls, as well as any changes in FDA policies, can affect the time and cost associated with the development, introduction and continued availability of new products. Where possible, we anticipate these factors in our product development processes. These agencies possess the authority to take various administrative and legal actions against us, such as product recalls, product seizures and other civil and criminal sanctions.
In order to reduce time and minimize the need to hire permanent technical and regulatory staffing in pursuing our FDA clearances we have contracted with Ximedica to prepare our Products for FDA submission. This is the most efficient way to maximize our chances for timely FDA clearances.
Based on all available data and opinions from our well qualified external consultants who specialize in FDA submissions, we believe that both our initial products and the follow-on products qualify for the 510(k) clearance path.
Foreign Regulation
As we plan to market our products in the EU and other foreign markets, in addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products in foreign countries. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Preparations for FDA Clearance Submissions and Design for Manufacturing
Up to this point, we have focused primarily on research and development of our first two Products. At this time, we have fully functional versions of our first two Products which we have used in most of our medical studies. We consider them to be (pre-production versions) as they need to go through a full FDA class improvement and testing cycle before they can be presented to the FDA for a 510(k) clearance. We are not yet at a stage to commence volume production of our products.
We have a contract with Ximedica to prepare our ER Product for clearance via a 510(K) submission to the FDA. The principal terms of this contract are to incorporate the product requirements, user needs, and 3D vector algorithm plugin developed by HeartBeam into the ER Myocardial Infarction software product. The contract with Ximedica, who has 35 years of medical device product development experience, is to be the development partner to turn the prototype into a commercially ready product cleared by the FDA. The principal terms of the agreement require Ximedica to perform the design, development, and testing of the software architecture for the product. This work includes designing subsystems for the user interface, thin client, cloud platform, and Electronic Medical Record interface. With oversight from and in partnership with HeartBeam, Ximedica will define all software requirements, software code, software unit testing, software detailed design, software verification protocols, and software test cases for all software subsystems included in the product. Once the subsystems are complete, Ximedica will perform system-level software testing. Once completed, the product will be provided to HeartBeam to perform system validation testing using clinical data from post-MI patients.
We have a scalable manufacturing strategy, and we have engaged this same firm, Ximedica, a firm with deep experience in developing FDA regulated products and contract manufacturing. In this regard, for the telehealth Product, we have an outline of a contract and a verbal agreement with Ximedica as well to prepare the Product for FDA 510(k) clearance submission.
59
Based on our extensive research and reference checking we believe that Ximedica is a US-based medical device and design organization that is FDA Registered and ISO 13485 Certified and has over 30 years of product development experience in medical devices and with manufacturing capabilities that we intend to use in the limited market release of our telehealth Generation 1 Product. We will consider other manufacturers, inside and outside of the US, for our high-volume manufacturing.
Properties and Facilities
We currently do not have any material real property leases.
Legal Proceedings
From time to time, we may be involved in various disputes and litigation matters that arise in the ordinary course of business. We are currently not a party to any material legal proceedings.
60
The following table and biographical summaries set forth information, including principal occupation and business experience, about our directors and executive officers as of the date of this prospectus:
|
Name |
Age |
Positions |
||
|
Richard Ferrari |
67 |
Executive Chairman of the Board of Directors |
||
|
Branislav Vajdic |
67 |
Chief Executive Officer, Director |
||
|
Richard Brounstein |
71 |
Chief Financial Officer |
||
|
Jon Hunt |
66 |
Executive Vice President and Chief Business Officer |
||
|
Willem Elfrink |
69 |
Director |
||
|
Marga Ortigas-Wedekind |
59 |
Director |
||
|
George A. de Urioste |
66 |
Director |
Richard Ferrari - Executive Chairman of the Board of Directors
Richard Ferrari, 67, joined our Board in 2019 and was appointed Executive Director of the Board of Directors in June 2021. Mr. Ferrari combines over 40 years of experience in Medical Device Start-ups as CEO, and entrepreneur. Also Mr. Ferrari is co-founder of De Novo Ventures which has $650M under management and has been Managing Director since 2000. Mr. Ferrari has also co-founded 6 more companies, two of which have been successful IPO’s and subsequent acquisitions, CTS one of the companies he co-founded was the fastest start-up to an IPO in the last 22 years in the medical device industry. Mr. Ferrari most recently from 2018 to 2021 was Chairman and CEO of PQ Bypass which was recently acquired by Endologix. Mr. Ferrari sits on the board of Pulmonx, a public company and is the Chairman of the Compensation Committee. Additionally, he is Executive Chairman of Tenon Medical, Vice-Chairman of ABS Interventional, Executive Chairman of Medlumics, and holds board positions with several other medical device start-ups. Mr. Ferrari has an undergraduate degree from Ashland University and an MBA from University of South Florida.
Branislav Vajdic, PhD - Chief Executive Officer and Director
Branislav Vajdic, PhD, 67, Chief Executive Officer and Founder of HeartBeam, Inc, combines over 30 years of experience in technology development and senior management positions. Dr. Vajdic has been deeply involved with the development of HeartBeam’s technology to fit his vision for the Company. Prior to HeartBeam from 2007 to 2010, Dr. Vajdic was CEO and Founder of NewCardio, a publicly traded company in the cardiovascular devices space. From 1984 to 2007, Dr. Vajdic was at Intel, where he held various senior management position. At Intel, Dr. Vajdic and was the designer of first Flash memory and two key inventions that enabled Flash as a product and led engineering groups responsible for Pentium 1 through Pentium 4 designs. Dr. Vajdic was awarded two Intel Achievement Awards, the highest level of award for outstanding contributions to Intel. Dr. Vajdic is author of numerous patents and publications in the fields of cardiovascular devices as well as chip design. Dr. Vajdic holds a PhD degree in Electrical Engineering from the University of Minnesota.
Richard Brounstein - Chief Financial Officer
Richard D. Brounstein, 71, Chief Financial Officer, combines over 30 years of experience in health technology senior management. Since 2017 Mr. Brounstein has been and is currently a partner of Hardesty, LLC, a financial services firm, and Mr. Brounstein is currently a managing director of CTRLCFO, LLC, a firm Mr. Brounstein founded in 2016 to support funded start-ups in life science and technology. Previously, from 2008 to 2011, Mr. Brounstein was Chief Financial Officer and Secretary on a part-time basis of NewCardio, Inc., a microcap public company in the Cardiology space, and over his career has been with nine other companies in life science or technology, holding positions including Chief Financial Officer, Chief Operating Officer, Treasurer and Accounting Manager. From June 2001 through November 2007, Mr. Brounstein held several positions at Calypte Biomedical Corporation, a publicly traded medical device company, including Chief Financial Officer and Executive Vice President. Mr. Brounstein served on the Board of CIMR (California Institute of Medical Research) from 2012 to 2018. Since 2000 Mr. Brounstein has been on the Board of Financial Executives International (FEI)’s Silicon Valley Chapter. In January 2007, Mr. Brounstein was appointed as the National Member Representative for the 2007 COSO Monitoring Project, which published new guidelines for monitoring internal financial controls in February 2009; Mr. Brounstein subsequently was a member of the FEI task force that issued the updated COSO Internal Control Framework in 2013. In March 2005, Mr. Brounstein
61
was appointed to the SEC Advisory Committee on Smaller Public Companies. Mr. Brounstein earned his Certified Public Accountant (CPA) certification while working at Arthur Andersen LLP, formerly a public accounting firm. Mr. Brounstein holds a B.A. in accounting and an M.B.A. in finance, both from Michigan State University.
Jon Hunt, Ph.D. - Executive Vice President and Chief Business Officer
Jon Hunt, 66, has over 34 years’ experience in the Medical/Medical Device Industry with extensive domestic and international experience in general management, clinical/regulatory, sales and marketing. He also has diverse experience in Fortune 500 companies as well as start-up environments. Dr. Hunt was the Vice President of Clinical Science and Technology, Medical Device Innovation Consortium from July 2019 to July 2021, Vice President of Clinical and Regulatory Affairs, Cryterion Medical from January 2018 to June 2019 (acquired by Boston Scientific Corporation in July 2018 for $202M). Dr. Hunt was the Founding President and CEO of Bardy Diagnostics, Inc. from October 2013 to November 2017 (acquired by Hill-Rom Holdings, Inc.). Prior to joining Bardy Diagnostics, Jon spent the previous 11 years as the Vice President of Clinical & Regulatory Affairs with Cameron Health, Inc. (acquired by Boston Scientific Corporation). Dr. Hunt spent the previous 10 years with Cardiac Pacemakers, Inc., St. Jude Medical and Cardiac Pathways Corporation. Dr. Hunt began his career with Cardiac Pacemakers, Inc. (now Boston Scientific Corporation) as the Director of Clinical Programs; he subsequently held positions at St. Jude Medical in Clinical Affairs and as the Business Unit Director for the Cardiac Rhythm Management division for Europe, the Middle East and Africa. At Cardiac Pathways Corporation, Dr. Hunt held various executive positions as Vice President of International Sales and Marketing and Vice President of Worldwide Sales and Marketing (acquired by Boston Scientific Corporation). Dr. Hunt received his Ph.D. in Motor Control from The Pennsylvania State University, his Master’s from California State University, Long Beach and his undergraduate degree from Keele University in the United Kingdom.
Willem Elfrink - Director
Willem Elfrink, 69, was Chairman of the Board since our founding and in June 2021 stepped down from this position but remains a Board member. Mr. Elfrink has over 40 years of experience in bringing new technologies to the market. Mr. Elfrink actively contributes to portfolio companies via board participation, strategic marketing, governance and capital structure. Mr. Elfrink is also the Founder and President of WPE Ventures Digitized Solutions, a security and digitization solutions investment firm. Mr. Elfrink joined Cisco in 1997 and was Cisco’s Executive Vice President of Industry Solutions and Chief Globalization Officer from 2000 to 2006 and 2007 to 2015 respectively, where Mr. Elfrink made and contributed to key strategic and operational decisions of the Company. Widely recognized as Cisco’s Corporate Entrepreneur in residence, his global charter was to identify significant technology opportunities. Mr. Elfrink also led an industry initiative — called the Internet of Things World Forum (IOTWF). Before joining Cisco, Mr. Elfrink held management and senior management positions at Olivetti, Xerox, HP, Digital Equipment Corporation (DEC) and Philips. Mr. Elfrink earned a Bachelor of Engineering degree from the Institute of Technology in Rotterdam, the Netherlands.
Marga Ortigas-Wedekind - Director
Marga Ortigas-Wedekind, 59, Board member combines over 30 years of experience in health technology senior management. Ms. Ortigas-Wedekind has been Chief Commercial Officer of Fogarty Innovation, a non-profit educational incubator for early stage medtech companies since December 2019. From July 2015 through July 2019, Ms. Ortigas-Wedekind was the Executive Vice President of Marketing and Payer Relations for iRhythm Technologies Inc., a publicly-traded digital healthcare company. From 2009 to 2015, Ms. Ortigas-Wedekind was Executive Vice President, Global Marketing and Product Development of Omnicell Inc., a publicly-traded developer of automated medication dispensing and analytics systems where she led the Marketing, International and Engineering departments. From 2002 to December 2008, Ms. Ortigas-Wedekind was the Senior Vice President, Marketing, Development, and Clinical Affairs and Xoft, Inc, a developer of disruptive technology to deliver radiation therapy with capital equipment and high-end disposables. Ms. Ortigas-Wedekind is on the board of Itamar Medical (NASDAQ: ITMR), which provides digitally-enabled systems for sleep apnea management, Total Flow Cannula, an early stage company developing a mechanism to improve safety during on-pump open heart surgery and, the Bay Area Cancer Coalition, a non-profit organization that supports those affected by breast or ovarian cancer. Ms. Ortigas-Wedekind is a limited partner and advisory board member for Launchpad digital health, a venture fund focused on digital heath technologies and is also an angel investor with Health Tech Capital. Ms. Ortigas-Wedekind has an undergraduate degree from Wellesley College and an MBA from the Stanford Graduate School of Business.
62
George A. de Urioste - Director
Mr. de Urioste, 66, combines over 30 years of experience in High Technology industry senior management. Previously, he had been involved in over 10 companies, holding positions including Board Director, Chief Operating Officer and Chief Financial Officer. From 1992 to 1998, he was Chief Financial Officer of Remedy Corporation (software), from 2000 to 2003, he was Chief Executive Officer of Aeroprise, Inc. (software), from 2004 to 2006 he was Chief Operating Officer and Chief Financial Officer for Chordiant Software, Inc. (software) In 2008, he was interim Chief Operating Officer and Chief Financial Officer for Marvell Technology, Inc.(semiconductors). From 2014 through 2018, he was Chief Financial Officer for Pluribus Networks, Inc. (software). From 2019 to 2020 he was Chief Financial Officer for 4iQ, Inc. (software) and is currently interim Chief Financial Officer for Mozilla, Inc. (software). Mr. de Urioste’s Board Director experience includes Audit Committee chairman roles for the following companies: Rainmaker Systems, Inc. (business outsourcing), from 2003 to 2005, Saba Software, Inc. (software) from 2008 to 2010, GCT, Inc. (semiconductors), from 2009 to 2011 Villa Montalvo (performing arts center), from 2011 to 2013, Bridgelux, Inc., from 2011 to 2016 (LED lighting), and Vendavo, Inc., from 2013 to 2014 (software). He was also chairman of the Board of Directors for Aeroprise, Inc. from 2000 to 2005 (software). He is currently a Board Director at Silicon Valley Directors Exchange, (a not-for-profit for Board education events). He has an undergraduate degree from University of Southern California and an MBA from University of California at Berkeley. Mr. de Urioste is also a Certified Public Accountant (inactive) in the State of California.
Director Terms; Qualifications
Members of our board of directors serve until the next annual meeting of stockholders, or until their successors have been duly elected.
When considering whether directors and nominees have the experience, qualifications, attributes and skills to enable the board of directors to satisfy its oversight responsibilities effectively in light of the Company’s business and structure, the board of directors focuses primarily on the industry and transactional experience, and other background, in addition to any unique skills or attributes associated with a director.
Director or Officer Involvement in Certain Legal Proceedings
There are no material proceedings to which any director or officer, or any associate of any such director or officer, is a party that is adverse to our Company or any of our subsidiaries or has a material interest adverse to our Company or any of our subsidiaries. No director or executive officer has been a director or executive officer of any business which has filed a bankruptcy petition or had a bankruptcy petition filed against it during the past ten years. No director or executive officer has been convicted of a criminal offense or is the subject of a pending criminal proceeding during the past ten years. No director or executive officer has been the subject of any order, judgment or decree of any court permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities during the past ten years. No director or officer has been found by a court to have violated a federal or state securities or commodities law during the past ten years.
Directors and Officers Liability Insurance
The Company plans on obtaining directors’ and officers’ liability insurance insuring its directors and officers against liability for acts or omissions in their capacities as directors or officers, subject to certain exclusions. Such insurance may also insure the Company against losses, which it may incur in indemnifying its officers and directors. In addition, officers and directors also have indemnification rights under applicable laws, and the Company’s Articles of Incorporation and Bylaws.
Director Independence
The listing rules of The Nasdaq Stock Market LLC (“Nasdaq”) require that independent directors must comprise a majority of a listed company’s board of directors. In addition, the rules of Nasdaq require that, subject to specified exceptions, each member of a listed company’s audit, compensation, and nominating and governance committees be independent. Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. Under the rules of Nasdaq, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
63
Our board of directors has undertaken a review of the independence of our directors and considered whether any director has a material relationship with it that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, the board of directors has determined that three are “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing standards of Nasdaq. In making these determinations, our board of directors considered the current and prior relationships that each non-employee director has with the Company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of the Company’s capital stock by each non-employee director, and any transactions involving them described in the section captioned “— Certain relationships and related transactions and director independence.”
Board Committees
The Company’s Board has established three standing committees: Audit, Compensation, and Nominating and Corporate Governance. Each of the committees will operate pursuant to its charter. The committee charters will be reviewed annually by the Nominating and Corporate Governance Committee. If appropriate, and in consultation with the chairs of the other committees, the Nominating and Corporate Governance Committee may propose revisions to the charters. The responsibilities of each committee are described in more detail below.
Nasdaq permits a phase-in period of up to one year for an issuer registering securities in an initial public offering to meet the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee independence requirements. Under the initial public offering phase-in period, only one member of each committee is required to satisfy the heightened independence requirements at the time our registration statement becomes effective, a majority of the members of each committee must satisfy the heightened independence requirements within 90 days following the effectiveness of our registration statement, and all members of each committee must satisfy the heightened independence requirements within one year from the effectiveness of our registration statement.
Audit Committee
The Audit Committee, among other things, will be responsible for:
• appointing; approving the compensation of; overseeing the work of; and assessing the independence, qualifications, and performance of the independent auditor;
• reviewing the internal audit function, including its independence, plans, and budget;
• approving, in advance, audit and any permissible non-audit services performed by our independent auditor;
• reviewing our internal controls with the independent auditor, the internal auditor, and management;
• reviewing the adequacy of our accounting and financial controls as reported by the independent auditor, the internal auditor, and management;
• overseeing our financial compliance system; and
• overseeing our major risk exposures regarding the Company’s accounting and financial reporting policies, the activities of our internal audit function, and information technology.
The board of directors has affirmatively determined that each member of the Audit Committee meets the additional independence criteria applicable to audit committee members under SEC rules and Nasdaq listing rules. Effective upon the completion of this offering the board of directors will adopt a written charter setting forth the authority and responsibilities of the Audit Committee. The Board has affirmatively determined that each member of the Audit Committee is financially literate, and that Mr. de Urioste meets the qualifications of an Audit Committee financial expert.
The Audit Committee will consist of Mr. de Urioste, Ms. Ortigas-Wedekind and Mr. Elfrink. Mr. de Urioste will chair the Audit Committee. We believe that, after consummation of this offering, the functioning of the Audit Committee will comply with the applicable requirements of the rules and regulations of the Nasdaq listing rules and the SEC.
64
Compensation Committee
The Compensation Committee will be responsible for:
• reviewing and making recommendations to the Board with respect to the compensation of our officers and directors, including the CEO;
• overseeing and administering the Company’s executive compensation plans, including equity-based awards;
• negotiating and overseeing employment agreements with officers and directors; and
• overseeing how the Company’s compensation policies and practices may affect the Company’s risk management practices and/or risk-taking incentives.
Effective upon the completion of this offering, the board of directors will adopt a written charter setting forth the authority and responsibilities of the Compensation Committee.
The Compensation Committee will consist of Mr. Ferrari, Mr. Elfrink and Mr. de Urioste, Mr. Ferrari will serve as chairman of the Compensation Committee. The board of directors has affirmatively determined that each member of the Compensation Committee meets the independence criteria applicable to compensation committee members under SEC rules and Nasdaq listing rules. The Company believes that, after the consummation of the offering, the composition of the Compensation Committee will meet the requirements for independence under, and the functioning of such Compensation Committee will comply with, any applicable requirements of the rules and regulations of Nasdaq listing rules and the SEC.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee, among other things, will be responsible for:
• reviewing and assessing the development of the executive officers and considering and making recommendations to the Board regarding promotion and succession issues;
• evaluating and reporting to the Board on the performance and effectiveness of the directors, committees and the Board as a whole;
• working with the Board to determine the appropriate and desirable mix of characteristics, skills, expertise and experience, including diversity considerations, for the full Board and each committee;
• annually presenting to the Board a list of individuals recommended to be nominated for election to the Board;
• reviewing, evaluating, and recommending changes to the Company’s Corporate Governance Principles and Committee Charters;
• recommending to the Board individuals to be elected to fill vacancies and newly created directorships;
• overseeing the Company’s compliance program, including the Code of Conduct; and
• overseeing and evaluating how the Company’s corporate governance and legal and regulatory compliance policies and practices, including leadership, structure, and succession planning, may affect the Company’s major risk exposures.
Effective upon completion of this offering., the board of directors will adopt a written charter setting forth the authority and responsibilities of the Corporate Governance/Nominating Committee.
The Nominating and Corporate Governance Committee will consist of Ms. Ortigas-Wedekind and Mr. de Urioste. Ms. Ortigas-Wedekind will serve as chairperson. The Company’s board of directors has determined that each member of the Nominating and Corporate Governance Committee is independent within the meaning of the independent director guidelines of Nasdaq listing rules.
65
Compensation Committee Interlocks and Insider Participation
None of the Company’s executive officers serves, or in the past has served, as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any entity that has one or more executive officers who serve as members of the Company’s board of directors or its compensation committee. None of the members of the Company’s compensation committee is, or has ever been, an officer or employee of the company.
Code of Business Conduct and Ethics
Prior to the completion of this offering, the Company’s Board of Directors will adopt a code of business conduct and ethics applicable to its employees, directors and officers, in accordance with applicable U.S. federal securities laws and the corporate governance rules of Nasdaq. The code of business conduct and ethics will be publicly available on the Company’s website. Any substantive amendments or waivers of the code of business conduct and ethics or code of ethics for senior financial officers may be made only by the Company’s board of directors and will be promptly disclosed as required by applicable U.S. federal securities laws and the corporate governance rules of Nasdaq.
Corporate Governance Guidelines
Prior to the completion of this offering, the Company’s board of directors will adopt corporate governance guidelines in accordance with the corporate governance rules of Nasdaq.
66
Summary Compensation Table
|
Name and Principle Position |
Year |
Salary |
Stock |
Total |
|||||||
|
Branislav Vajdic |
2020 |
$ |
81,856 |
$ |
— |
$ |
81,856 |
||||
|
CEO and Director(2) |
2019 |
$ |
62,147 |
$ |
— |
$ |
62,147 |
||||
|
|
|
|
|||||||||
|
Richard Brounstein |
2020 |
$ |
35,954 |
$ |
— |
$ |
35,954 |
||||
|
CFO |
2019 |
$ |
— |
$ |
10 |
$ |
10 |
||||
____________
(1) Represents the full grant date fair value of the stock award or option grant, as applicable, calculated in accordance with FASB ASC Topic 718 and FASB ASC 505, Equity-Based Payments to Non-employees. Our policy and assumptions made in the valuation of share-based payments are contained in Note 6 to our December 31, 2020 financial statements. The value of stock awards presented in the Summary Compensation Table reflects the grant date fair value of the awards and does not correspond to the actual value that will be recognized by the named executive officers.
(2) Does not include the unvested service warrants issued to Dr. Vajdic in February 2019, which are subject to certain vesting requirements.
Employment Agreements
As of September 30, 2021, the Company has three employment agreements, Branislav Vajdic, the Company’s chief executive officer, Richard Brounstein the Company’s chief financial officer and Jon Hunt the Company’s Vice President and Chief Business Officer.
Branislav Vajdic Employment Agreement
The company entered into an employment agreement dated September 10, 2021 with Dr. Vajdic as its Chief Executive Officer and a member of the board of directors. Dr. Vajdic will receive an annual salary of $325,000, commencing on September 15, 2021, which will be paid semi monthly in accordance with the Company’s normal payroll procedures. Dr. Vajdic will also be eligible to receive certain employee benefits and bonuses under a bonus plan program which will be established upon the closing of this offering.
Richard Brounstein Employment Agreement
The company entered into an employment agreement dated September 10, 2021 with Richard Brounstein as its Chief Financial Officer. Mr. Brounstein will receive an annual salary of $187,000, commencing on September 15, 2021, which will be paid semi monthly in accordance with the Company’s normal payroll procedures. Mr. Brounstein will also be eligible to receive certain employee benefits and bonuses under a bonus plan program which will be established upon the closing of this offering.
Jon Hunt Employment Agreement
The company entered into an employment agreement dated September 10, 2021 with Jon Hunt as its Vice President and Chief Business Officer. Dr. Hunt will receive an annual salary of $275,000, commencing on September 15, 2021, which will be paid semi monthly in accordance with the Company’s normal payroll procedures. Dr. Hunt will also be eligible to receive certain employee benefits and bonuses under a bonus plan program which will be established upon the closing of this offering.
67
Outstanding Equity Awards at Fiscal Year-End 2020
At December 31, 2020, we had outstanding equity awards as follows:
|
Name |
Number of |
Number of |
Option |
Option |
Equity: |
Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested |
||||||||
|
Branislav Vajdic(1) |
— |
— |
|
— |
— |
363,636 |
$ |
109 |
||||||
|
Richard Brounstein(2) |
— |
18,181 |
$ |
0.0003 |
2/10/2029 |
— |
|
— |
||||||
____________
(1) Dr. Vajdic was awarded a service warrant to acquire 363,636 shares of Common Stock at a per share price of $0.0003. See F-13 for a description of the terms. They are unvested on December 31, 2020 and expire February 12, 2023.
(2) Mr. Brounstein was awarded 36,363 options with the option to early exercise on February 11, 2019, these options are scheduled to vest over 4 years monthly and are 50% vested at December 31, 2020. 26,515 shares are vested and included in common stock outstanding and 9,848 shares remain unvested within 60 days of September 30, 2020. The option exercise price is $0.0003 per share.
Director Compensation
The following table presents the total compensation for each person who served as a non-employee member of our board of directors and received compensation for such service during the fiscal year ended December 31, 2020. Other than as set forth in the table and described more fully below, we did not pay any compensation, make any equity awards or non-equity awards to, or pay any other compensation to any of the non-employee members of our board of directors in 2020.
|
Name |
Fees Earned or |
Option Awards |
Non-Equity |
Total |
||||||||
|
Richard Ferrari(2) |
$ |
— |
$ |
4,500 |
$ |
— |
$ |
4,500 |
||||
|
Marga Ortigas-Wedekind(3) |
$ |
— |
$ |
4,500 |
$ |
— |
$ |
4,500 |
||||
____________
(1) Represents the full grant date fair value of the 27,272 each or option grant, calculated in accordance with FASB ASC Topic 718 and FASB ASC 505, Equity-Based Payments to Non-employees. These options were outstanding on December 31, 2020. Our policy and assumptions made in the valuation of share-based payments are contained in Note 6 to our December 31, 2020 financial statements. The value of stock awards presented in the Summary Compensation Table reflects the grant date fair value of the awards and does not correspond to the actual value that will be recognized by the named executive officers.
(2) Mr. Ferrari’s 27,272 share option grant in 2020 plus a 2021 grant of 181,819 shares represents 36,364 shares exercisable within 60 days after September 30, 2021, and 172,727 unvested stock options.
(3) Ms. Ortigas-Wedekind’s 27,272 share option grant in 2020 plus a 2021 grant of 16,364 shares represents 15,682 shares exercisable within 60 days after September 30, 2021, and 27,954 unvested stock options.
68
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has no public market for the Company’s Common Stock and Warrants, and a liquid trading market for its Common Stock and Warrants may not develop or be sustained after this offering. Future sales of substantial amounts of the Company’s Common Stock and Warrants in the public market, or the anticipation of these sales, could materially and adversely affect market prices prevailing from time to time, and could impair the Company’s ability to raise capital through sales of equity or equity-related securities.
Only a limited number of shares of the Company’s Common Stock and Warrants will be available for sale in the public market for a period of several months after completion of this offering due to contractual and legal restrictions on resale described below. Nevertheless, sales of a substantial number of shares of the Company’s Common Stock and Warrants in the public market after such restrictions lapse, or the perception that those sales may occur, could materially and adversely affect the prevailing market price of its Common Stock and Warrants. Although the Company intends to list its Common Stock and Warrants on The Nasdaq Capital Market, the Company cannot assure you that there will be an active market for its Common Stock and Warrants.
Of the shares to be outstanding immediately after the completion of this offering, we expect that the shares to be sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act; these restricted securities may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 under the Securities Act.
Rule 144
In general, under Rule 144 as currently in effect, once the Company has been subject to the public company reporting requirements of Section 13 or Section 15(d) of the Exchange Act for at least 90 days, a person who is not deemed to have been one of the Company’s affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares of its Common Stock proposed to be sold for at least six months is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than Company affiliates, then that person would be entitled to sell those shares without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, the Company’s affiliates or persons selling shares of its Common Stock on behalf of its affiliates are entitled to sell upon expiration of the market standoff agreements and lock-up agreements described above, within any three-month period, a number of shares that does not exceed the greater of:
(a) 1% of the number of shares of the Company’s capital stock then outstanding; or
(b) the average weekly trading volume of the Company’s Common Stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale.
Sales under Rule 144 by the Company’s affiliates or persons selling shares of its Common Stock on behalf of its affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about the Company.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of the Company’s Common Stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of the Company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation, or notice provisions of Rule 144. Rule 701 also permits affiliates of the Company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701 and until expiration of the lock-up period described below.
69
Lock-Up Agreements
In connection with this offering, the Company, and its officers, directors and all stockholders and convertible note holders have agreed to a six-month “lock-up” period from the closing of this offering, with respect to the shares that they beneficially own, including shares issuable upon the exercise of convertible securities and options that are currently outstanding or which may be issued. This means that, for a period of six months following the closing of this offering, such persons may not offer, sell, pledge or otherwise dispose of these securities without the prior written consent of the underwriters. Officers’ and Directors’ six-month restricted period is subject to extension upon certain events and the terms of the lock-up agreements may be waived at the underwriters’ discretion. The lock-up restrictions, specified exceptions and the circumstances under which the nine-month lock-up period may be extended are described in more detail under “Underwriting.”
70
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF THE COMPANY’S COMMON STOCK
The following is a summary of the material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the ownership and disposition of the Company’s Common Stock but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended, or the Internal Revenue Code, Treasury regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may be changed, possibly retroactively, so as to result in U.S. federal income tax consequences different from those set forth below. No ruling on the U.S. federal, state, or local tax considerations relevant to the Company’s operations or to the purchase, ownership or disposition of its shares, has been requested from the IRS or other tax authority. No assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to any of the tax consequences described below.
This summary also does not address the tax considerations arising under the laws of any non-U.S., state or local jurisdiction, or under U.S. federal gift and estate tax laws, except to the limited extent set forth below. In addition, this discussion does not address tax considerations applicable to an investor’s particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
• banks, insurance companies or other financial institutions, regulated investment companies or real estate investment trusts;
• persons subject to the alternative minimum tax or Medicare contribution tax on net investment income;
• tax-exempt organizations or governmental organizations;
• controlled foreign corporations, passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax;
• brokers or dealers in securities or currencies;
• traders in securities that elect to use a mark-to-market method of accounting for their securities holdings;
• persons that own, or are deemed to own, more than five percent of the Company’s capital stock (except to the extent specifically set forth below);
• U.S. expatriates and certain former citizens or long-term residents of the United States;
• partnerships or entities classified as partnerships for U.S. federal income tax purposes or other pass-through entities (and investors therein);
• persons who hold the Company’s Common Stock as a position in a hedging transaction, “straddle,” “conversion transaction” or other risk reduction transaction or integrated investment;
• persons who hold or receive the Company’s Common Stock pursuant to the exercise of any employee stock option or otherwise as compensation;
• persons who do not hold the Company’s Common Stock as a capital asset within the meaning of Section 1221 of the Internal Revenue Code; or
• persons deemed to sell the Company’s Common Stock under the constructive sale provisions of the Internal Revenue Code.
In addition, if a partnership or entity classified as a partnership for U.S. federal income tax purposes holds the Company’s Common Stock, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold the Company’s Common Stock, and partners in such partnerships, should consult their tax advisors.
71
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership and disposition of the Company’s Common Stock arising under the U.S. federal estate or gift tax rules or under the laws of any state, local, non-U.S., or other taxing jurisdiction or under any applicable tax treaty.
Non-U.S. Holder Defined
For purposes of this discussion, you are a non-U.S. holder (other than a partnership) if you are any holder other than:
• an individual citizen or resident of the United States (for U.S. federal income tax purposes);
• a corporation or other entity taxable as a corporation created or organized in the United States or under the laws of the United States, any state thereof, or the District of Columbia, or other entity treated as such for U.S. federal income tax purposes;
• an estate whose income is subject to U.S. federal income tax regardless of its source; or
• a trust (x) whose administration is subject to the primary supervision of a U.S. court and which has one or more “U.S. persons” (within the meaning of Section 7701(a)(30) of the Internal Revenue Code) who have the authority to control all substantial decisions of the trust or (y) which has made a valid election to be treated as a U.S. person.
Distributions
As described in “Dividend Policy,” the Company has never declared or paid cash dividends on its Common Stock and do not anticipate paying any dividends on its Common Stock in the foreseeable future. However, if the Company does make distributions on its Common Stock, those payments will constitute dividends for U.S. tax purposes to the extent paid from the Company’s current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both the Company’s current and its accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in the Company’s Common Stock, but not below zero, and then will be treated as gain from the sale of stock as described below under “— Gain on Disposition of Common Stock.”
Subject to the discussion below on effectively connected income, backup withholding and foreign accounts, any dividend paid to you generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. In order to receive a reduced treaty rate, you must provide us with an IRS Form W-8BEN, IRS Form W-8BEN-E, or other appropriate version of IRS Form W-8 certifying qualification for the reduced rate. A non-U.S. holder of shares of the Company’s Common Stock eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, which then will be required to provide certification to the Company or its paying agent, either directly or through other intermediaries.
Dividends received by you that are effectively connected with your conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, attributable to a permanent establishment maintained by you in the United States) are generally exempt from the withholding tax described above. In order to obtain this exemption, you must provide us with an IRS Form W-8ECI or other applicable IRS Form W-8 properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. In addition, if you are a corporate non-U.S. holder, dividends you receive that are effectively connected with your conduct of a U.S. trade or business may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. You should consult your tax advisor regarding any applicable tax treaties that may provide for different rules.
72
Gain on Disposition of Common Stock
Subject to the discussion below regarding backup withholding and foreign accounts, you generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of the Company’s Common Stock unless:
• the gain is effectively connected with your conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, the gain is attributable to a permanent establishment maintained by you in the United States);
• you are a non-resident alien individual who is present in the United States for a period or periods aggregating 183 days or more during the taxable year in which the sale or disposition occurs and certain other conditions are met; or
• the Company’s Common Stock constitutes a United States real property interest by reason of its status as a “United States real property holding corporation,” or USRPHC, for U.S. federal income tax purposes at any time within the shorter of (i) the five-year period preceding your disposition of the Company’s Common Stock, or (ii) your holding period for its Common Stock.
The Company believes that it is not currently and will not become a USRPHC for U.S. federal income tax purposes, and the remainder of this discussion so assumes. However, because the determination of whether it is a USRPHC depends on the fair market value of its U.S. real property relative to the fair market value of its other business assets, there can be no assurance that the Company will not become a USRPHC in the future. Even if it becomes a USRPHC, however, as long as the Company’s Common Stock is regularly traded on an established securities market, such Common Stock will be treated as U.S. real property interests only if you actually or constructively hold more than five percent of such regularly traded Common Stock at any time during the shorter of (i) the five-year period preceding your disposition of the Company’s Common Stock, or (ii) your holding period for the Company’s Common Stock.
If you are a non-U.S. holder described in the first bullet above, you will be required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates, and a corporate non-U.S. holder described in the first bullet above also may be subject to the branch profits tax at a 30% rate, or such lower rate as may be specified by an applicable income tax treaty. If you are an individual non-U.S. holder described in the second bullet above, you will be required to pay a flat 30% tax (or such lower rate specified by an applicable income tax treaty) on the gain derived from the sale, which gain may be offset by U.S. source capital losses for the year (provided you have timely filed U.S. federal income tax returns with respect to such losses). You should consult any applicable income tax or other treaties that may provide for different rules.
Backup Withholding and Information Reporting
Generally, the Company must report annually to the IRS, regardless of whether any tax was withheld, the amount of dividends paid to you, your name and address and the amount of tax withheld, if any. A similar report will be sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence.
Payments of dividends or of proceeds on the disposition of stock made to you may be subject to information reporting and backup withholding at a current rate of 24% unless you establish an exemption, for example, by properly certifying your non-U.S. status on an IRS Form W-8BEN, IRS Form W-8BEN-E, or another appropriate version of IRS Form W-8.
Backup withholding is not an additional tax; rather, the U.S. federal income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner.
73
Foreign Account Tax Compliance
The Foreign Account Tax Compliance Act, or FATCA, imposes withholding tax at a rate of 30% on dividends on and gross proceeds from the sale or other disposition of the Company’s Common Stock paid to “foreign financial institutions” (as specially defined under these rules), unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding the U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise establishes an exemption. FATCA also generally imposes a U.S. federal withholding tax of 30% on dividends on and gross proceeds from the sale or other disposition of the Company’s Common Stock paid to a “non-financial foreign entity” (as specially defined for purposes of these rules) unless such entity provides the withholding agent with a certification identifying certain substantial direct and indirect U.S. owners of the entity, certifies that there are none or otherwise establishes an exemption. The withholding provisions under FATCA generally apply to dividends on our Common Stock, and under current transition rules, are expected to apply with respect to the gross proceeds from the sale or other disposition of the Company’s Common Stock on or after January 1, 2019. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult their own tax advisors regarding the possible implications of this legislation on their investment in the Company’s Common Stock.
Each prospective investor should consult its own tax advisor regarding the particular U.S. federal, state and local and non-U.S. tax consequences of purchasing, holding and disposing of the Company’s Common Stock, including the consequences of any proposed change in applicable laws.
74
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the Company’s Common Stock, beneficially owned as of September 30, 2021 (i) each person known to the Company to beneficially own more than 5% of its Common Stock, (ii) each executive officer, director and director nominee and (iii) all officers, directors and director nominees as a group. The following table is based on the Company having 3,555,326 shares of Common Stock issued and outstanding as of September 30, 2021. The Company calculated beneficial ownership according to Rule 13d-3 of the Securities Exchange Act of 1934, as amended as of that date. Shares of the Company’s Common Stock issuable upon exercise of options or warrants or conversion of notes that are exercisable or convertible within 60 days after September 30, 2021 are included as beneficially owned by the holder, but not deemed outstanding for computing the percentage of any other stockholder for Percentage of Common Stock Beneficially Owned. For each individual and group included in the table below, percentage ownership is calculated by dividing the number of shares beneficially owned by such person or group by the sum of the 3,555,326 shares of Common Stock outstanding at September 30, 2021, plus the number of shares of Common Stock that such person or group had the right to acquire on or within 60 days after September 30, 2021. Beneficial ownership generally includes voting and dispositive power with respect to securities. Unless otherwise indicated below, the persons and entities named in the table have sole voting and sole dispositive power with respect to all shares beneficially owned.
|
Shares Beneficially Owned |
Percentage Before Completion of Offering |
Percentage After Completion of Offering |
||||||
|
Richard Ferrari(1) |
101,450 |
1.01 |
% |
1.30 |
% |
|||
|
Branislav Vajdic(2) |
915,148 |
22.47 |
% |
11.79 |
% |
|||
|
Willem Pieter Elfrink(3) |
441,128 |
3.11 |
% |
5.68 |
% |
|||
|
Marga Ortigas-Wedekind(4) |
23,442 |
* |
|
* |
|
|||
|
George de Urioste |
— |
* |
|
* |
|
|||
|
Rick Brounstein(5) |
104,861 |
1.81 |
% |
1.35 |
% |
|||
|
Jon Hunt |
— |
* |
|
* |
|
|||
|
All current executive officers and directors as a group |
28.35 |
% |
20.27 |
% |
||||
|
5% or greater stockholders |
|
|
||||||
|
Bosko Bojovic |
517,273 |
14.55 |
% |
6.67 |
% |
|||
|
Mirjana Vajdic |
400,000 |
11.25 |
% |
5.16 |
% |
|||
|
Ljupco Hadzievski |
389,091 |
10.94 |
% |
5.02 |
% |
|||
|
Mile Boca |
312,727 |
8.80 |
% |
4.03 |
% |
|||
____________
* Less than 1 percent ownership
(1) Includes (i) 36,364 options exercisable within 60 days after September 30, 2021, (x) principal and interest on his 8% Convertible Notes investments totalling $273,312 (65,086 shares after completion of the offering) at September 30, 2021. Does not include 172,727 unvested stock options.
(2) Includes (i) 794,545 shares acquired as founding stock and (ii) 5,818 four-year warrants exercisable at $2.75/share and acquired as a result of a short-term loan investment program, (x) principal and interest on his 8% Convertible Notes investments totalling of $482,097 (114,785 shares after completion of the offering) at September 30, 2021, and (y) 363,636 unvested 2019 service warrants exercisable at $0.0003.
(3) Includes (i) 105,454 shares acquired through the 2015 Stock Incentive Plan, (ii) 3,640 four-year warrants exercisable at $2.75/share and acquired as a result of a short-term loan investment program and (iii) 1,818 options exercisable within 60 days after September 30, 2021, (x) principal and interest on his 8% Convertible Notes investments totalling of $1,386,907 (330,216 shares after completion of the offering) at September 30, 2021, (y) 43,636 unvested 2019 service warrants exercisable at $0.0003. Does not include 38,181 unvested stock options.
(4) Includes (i) 15,682 options exercisable within 60 days after September 30, 2021, (x) principal and interest on her 8% Convertible Notes investments totalling of $32,543 (7,760 shares after completion of the offering) at September 30, 2021. Does not include 27,954 unvested stock options.
(5) Includes (i) 61,363, shares acquired through the 2015 Stock Incentive Plan including 1,515 which vest within 60 days after September 30, 2021, and (ii) 1,455 four-year warrants exercisable at $2.75/share and acquired as a result of a short-term loan investment program, (x) principal and interest on his 8% Convertible Notes investments totalling of $170,218 (40,528 shares after completion of the offering) at September 30, 2021, of which $48,485 are held in the name of his son, Daniel Brounstein, and wife, Stephna May and 9,848 shares of Common Stock that are unvested within 60 days after September 30, 2021. Does not include 9,848 unvested stock options.
75
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following includes a summary of transactions during our quarterly periods ended June 30, 2021 and June 30, 2020 and our fiscal years ended December 31, 2020 and December 31, 2019 to which we have been a party, including transactions in which the amount involved in the transaction exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described elsewhere in this proxy statement. We are not otherwise a party to a current related party transaction, and no transaction is currently proposed, in which the amount of the transaction exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years and in which a related person had or will have a direct or indirect material interest.
During the course of business, we obtain accounting services from CTRLCFO, a firm in which the Chief Financial Officer of the Company has significant influence, as well as Hardesty, where he is a non-managing partner. We incurred approximately $55,000 and $8,000 in accounting fees from these firms during the six months ended June 30, 2021 and 2020, respectively. We incurred approximately $82,000 and $21,000 in accounting fees from these firms during the years ended December 31, 2020 and 2019, respectively. As of June 30, 2021, December 31, 2020, and December 31, 2019, the Company had balances due to these firms amounting to approximately $11,000, $15,000, and $2,000, respectively.
All of our Directors and Officers have invested in the 2015 Notes of the Company, as have several consultants who provide services.
As of June 30, 2021, December 31, 2020, and 2019, investments from Directors and Officers represents approximately $2,308,000, $1,797,000 and $1,636,000, respectively. As of June 30, 2021, December 31, 2020 and 2019, investments from consultants represents approximately $886,000, $661,000 and $483,000, respectively.
Our related parties are our Directors and Officers. The table below represents the detail of their investments that correspond to the periods as of June 30, 2021, December 31, 2020, and 2019, respectively.
|
Name |
Title |
June 30, |
December 31, |
December 31, |
|||||||
|
Rich Ferrari |
Executive Chairman |
|
268,000 |
|
— |
|
— |
||||
|
Branislav Vajdic |
Chief Executive Officer |
|
474,600 |
|
446,000 |
|
406,000 |
||||
|
Wim Elfrink |
Director |
|
1,366,200 |
|
1,218,000 |
|
1,136,000 |
||||
|
Marga Ortigas-Wedekind |
Director |
|
32,000 |
|
11,000 |
|
10,000 |
||||
|
Richard Brounstein |
Chief Financial Officer |
|
167,200 |
|
122,000 |
|
84,000 |
||||
|
$ |
2,308,000 |
$ |
1,797,000 |
$ |
1,636,000 |
||||||
76
In connection with this offering, we will enter into an underwriting agreement with The Benchmark Company as representative for the underwriters in this offering. Each underwriter named below has severally agreed to purchase from us, on a firm commitment basis, the number of Units, set forth opposite its name below, at the public offering price, less the underwriting discount set forth on the cover page of this prospectus.
|
Underwriter |
Number of |
|
|
The Benchmark Company, LLC |
|
|
|
Total |
2,750,000 |
The underwriters are committed to purchase all of the Units offered by us other than those covered by the option to purchase additional securities described below, if they purchase any such securities. The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
The Company has agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act of 1933, as amended, and to contribute to payments the underwriters may be required to make in respect thereof.
The underwriters are offering the Units, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Over-allotment Option
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 30 days after the date of this prospectus, permits the underwriters to purchase a maximum of 412,500 additional shares of Common Stock (15% of the shares sold in this offering) and/or Warrants to purchase Common Stock from us to cover over-allotments, if any, at a price per share equal to the public offering price per Unit less the underwriting discounts and commissions set forth on the cover of this prospectus in any combination thereof to cover over-allotments, if any. We will be obligated, pursuant to the option, to sell these additional shares of Common Stock and/or Warrants to the underwriters to the extent the option is exercised.
Discount
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
|
Per |
Total |
Total With |
|||||||
|
Public offering price(1) |
$ |
$ |
$ |
||||||
|
Underwriting discount (7%) |
$ |
$ |
$ |
||||||
|
Proceeds, before expenses, to us |
$ |
$ |
$ |
||||||
____________
(1) Consists of $ attributable to the Common Stock and $0.01 attributable to the Warrant included in the Unit.
The underwriters propose to offer the shares offered by us to the public at the public offering price per share set forth on the cover of this prospectus. In addition, the underwriters may offer some of the shares to other securities dealers at such price less a concession of $ per share.
77
The Company will pay the out-of-pocket accountable expenses of the underwriters in connection with this offering. The underwriting agreement, however, provides that in the event the offering is terminated, any advance expense deposits paid to the underwriters will be returned to the extent that offering expenses are not actually incurred in accordance with FINRA Rule 5110(g)(4)(A).
The Company has agreed to pay the underwriters’ a non-accountable expense allowance equal to 1% of the aggregate gross proceeds of this offering. The Company has also agreed to pay for a certain amount of the underwriter’s accountable expenses including actual accountable road show expenses for the offering; prospectus tracking and compliance software for the offering; the reasonable and documented fees and disbursements of the underwriter’s counsel up to an amount of $100,000; background checks of the Company’s officers and directors; preparation of bound volumes and cube mementos in such quantities as the underwriter may reasonably request; provided that these actual accountable expenses of the underwriter shall not exceed $132,500 in the aggregate, including the fees and disbursements of the underwriter’s counsel.
The Company estimates that the total expenses of the offering payable by us, excluding underwriting discounts and commissions, will be approximately $650,000.
We have been advised by the Representative that it proposes to offer the Units offered by us to the public at the public offering price per Unit set forth on the cover of this prospectus. In addition, the underwriters may offer some of the Units to other securities dealers at such price less a concession of $ per Unit. After the initial offering, the public offering price and concession to dealers may be changed.
Representative Warrants
Upon the closing of this offering, we have agreed to issue to the representative for the underwriters a five-year warrant to purchase up to 7% of the Common Stock underlying the Units sold by us in this offering. The Representative’s Warrants will be exercisable at a per share exercise price equal to $ (or 100% of the public offering price per Unit). The Representative’s Warrants will be exercisable at any time, and from time to time, in whole or in part, during the period from the effective date of the offering, which period shall not extend further than five years from the date of commencement of sales in this offering in compliance with Financial Industry Regulatory Authority, or FINRA, Rule 5110. The Representative’s Warrants are also exercisable on a cashless basis. The Representative’s Warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to FINRA Rule 5110. Except as permitted by Rule 5110, the representative for the underwriters (or permitted assignees under the Rule) will not sell, transfer, assign, pledge, or hypothecate the Representative’s Warrants or the securities underlying the Representative’s Warrants, nor will any of them engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the option or the underlying securities for a period of 180 days from the commencement of sales under this prospectus. The exercise price and number of securities upon exercise of the Warrants may be adjusted in certain circumstances including in the event of a share dividend, extraordinary cash dividend or our recapitalization, reorganization, merger or consolidation. However, the Representative’s Warrant exercise price or underlying shares will not be adjusted for issuances of shares of Common Stock at a price below the Representative’s Warrant exercise price.
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements
Pursuant to the underwriting agreement and certain “lock-up” agreements, the Company, its executive officers, directors and certain holders of the Company’s Common Stock and securities exercisable for or convertible into its Common Stock outstanding immediately upon the closing of this offering, have agreed, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling of any Common Stock or securities convertible into or exchangeable or exercisable for any Common Stock, whether currently owned or subsequently acquired, without the prior written consent of the underwriters, for a period of six (6) months from the date of effectiveness of the offering.
78
Right of First Refusal
We have granted the underwriter a right of first refusal, for a period of twelve (12) months from the closing of this offering, to act as lead or joint-lead investment banker, lead or joint-lead book runner and/or lead or joint placement agent at the underwriter’s discretion, for each and every future public and private equity, equity-linked or debt (excluding commercial bank debt) offering, including all equity linked financings during such twelve (12) month period, of the Company, or any successor to or subsidiary of the Company.
Electronic Offer, Sale and Distribution of Units
A prospectus in electronic format may be made available on the websites maintained by the underwriters, if any, participating in this offering and the underwriters participating in this offering may distribute prospectuses electronically. The underwriters may agree to allocate a number of Units for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriters that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the underwriters in their capacity as underwriters, and should not be relied upon by investors.
Stabilization
In connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
• Stabilizing transactions permit bids to purchase Common Stock and/or Warrants so long as the stabilizing bids do not exceed a specified maximum and are engaged in for the purpose of preventing or retarding a decline in the market price of the Common Stock and/or Warrants while the offering is in progress.
• Over-allotment transactions involve sales by the underwriters of shares of Common Stock and/or Warrants in excess of the number of shares of Common Stock and/or Warrants the underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares of Common Stock and/or Warrants over-allotted by the underwriters is not greater than the number of shares of Common Stock and/or Warrants that they may purchase in the over-allotment option. In a naked short position, the number of shares of Common Stock and/or Warrants involved is greater than the number of shares of Common Stock and/or Warrants in the over-allotment option. The underwriters may close out any short position by exercising their over-allotment option and/or purchasing shares of Common Stock and/or Warrants in the open market.
• Syndicate covering transactions involve purchases of shares of Common Stock and/or Warrants in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares of Common Stock and/or Warrants to close out the short position, the underwriters will consider, among other things, the price of shares of Common Stock and/or Warrants available for purchase in the open market as compared with the price at which they may purchase shares of Common Stock and/or Warrants through exercise of the over- allotment option. If the underwriters sell more shares of Common Stock and/or Warrants than could be covered by exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying shares of Common Stock and/or Warrants in the open market. A naked short position is more likely to be created if the underwriters are concerned that after pricing there could be downward pressure on the price of the shares of Common Stock and/or Warrants in the open market that could adversely affect investors who purchase in the offering.
• Penalty bids permits the underwriters to reclaim a selling concession from a syndicate member when the shares of Common Stock and/or Warrants originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
79
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of the shares of Common Stock and/or Warrants or preventing or retarding a decline in the market price of its shares of Common Stock and/or Warrants. As a result, the price of the Common Stock and/or Warrants in the open market may be higher than it would otherwise be in the absence of these transactions. Neither the Company nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of the Company’s Common Stock and/or Warrants. These transactions may be effected on the Nasdaq Capital Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive Market Making
In connection with this offering, the underwriters may engage in passive market making transactions in the Company’s Common Stock on The Nasdaq Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Other Relationships
The underwriters and their respective affiliates may, in the future provide various investment banking, commercial banking and other financial services for the Company and its affiliates for which they have received, and may in the future receive, customary fees. However, except as disclosed in this prospectus, the Company has no present arrangements with the underwriters for any further services.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
80
Authorized and Outstanding Capital Stock
The following description of the Company’s capital stock and provisions of its Articles of Incorporation and Bylaws are summaries and are qualified by reference to the Company’s Articles of Incorporation and Bylaws which are filed as exhibits to the registration statement of which this prospectus forms a part.
The Company is authorized to issue 20,000,000 shares of capital stock, par value $0.0001 per share.
As of June 30, 2021, the Company had outstanding 3,547,168 shares of Common Stock held by 31 shareholders of record.
Units Offered Hereby
Each Unit has an assumed offering price of $6.00 and consists of one share of Common Stock and one Warrant. Each whole Warrant entitles the holder thereof to purchase one share of our Common Stock at a price of $ per share, (which will not be less than % of the public offering price of one Unit) per whole Common Stock. The Common Stock and Warrants comprising the Units are immediately separable upon issuance and will be issued separately in this offering. No fractional Warrants will be issued.
Common Stock
The holders of the Company’s Common Stock are entitled to one vote per share. In addition, the holders of the Company’s Common Stock will be entitled to receive dividends ratably, if any, declared by the Company’s board of directors out of legally available funds; however, the current policy of the board of directors is to retain earnings, if any, for operations and growth. Upon liquidation, dissolution or winding-up, the holders of the Company’s Common Stock are entitled to share ratably in all assets that are legally available for distribution. The holders of the Company’s Common Stock have no pre-emptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of the Company’s Common Stock are subject to, and may be adversely affected by, the rights of the holders of any series of preferred stock, which may be designated solely by action of the board of directors and issued in the future.
Warrants
The exercise price of the Warrants is $ per share (with an exercise price no less than % of the public offering price of one Unit). Each Warrant is exercisable for one share of our Common Stock, subject to adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock as described herein. A holder may not exercise any portion of a warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% of the outstanding Common Stock after exercise, as such percentage ownership is determined in accordance with the terms of the Warrants, except that upon notice from the holder to us, the holder may waive such limitation up to a percentage, not in excess of 9.99%. Each warrant will be exercisable immediately upon issuance and will expire five (5) years after the initial issuance date. The terms of the Warrants will be governed by a warrant agreement, dated as of the effective date of this offering, between us and Vstock Transfer, as the Warrant agent.
Listing
We have applied to list our Common Stock and Warrants on the Nasdaq Capital Market upon our satisfaction of the exchange’s initial listing criteria. Upon approval to list our Common Stock and Warrants on the Nasdaq Capital Market and we anticipate that the shares of Common Stock and Warrants, will be listed on the Nasdaq Capital Market under the symbol “BEAT” and “BEATW” respectively. No assurance can be given that our application will be approved. If our Common Stock and Warrants are not approved for listing on the Nasdaq Capital Market, we will not consummate this offering.
Transfer Agent and Warrant Agent
The Company’s transfer agent and Warrant Agent is VStock Transfer with an address of 18 Lafayette Place Woodmere, New York 11598.
81
Indemnification of Directors and Officers
Each of our Articles of Incorporation and our Bylaws provide for indemnification of our directors and officers. Our Bylaws provide that we will indemnify any person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative (other than an action by or in the right of the corporation) by reason of the fact that such person is or was a director or officer of the corporation, against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the Company, and with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere or its equivalent will not, without more, create a presumption that the person did not act in good faith and in a manner which such person reasonably believed to be in or not opposed to the best interest of the corporation, and, with respect to any criminal action or proceeding, had reasonable cause to believe that his conduct was unlawful. The Company may by action of its Board of Directors, grant rights to indemnification and advancement of expenses to employees and agents of the Company with the same scope and effects as the indemnification provisions for officers and directors.
Disclosure of Commission Position on Indemnification for Securities Act Liabilities
Insofar as indemnification for liabilities under the Securities Act may be permitted to officers, directors or persons controlling the Company pursuant to the foregoing provisions, the Company has been informed that is it is the opinion of the Securities and Exchange Commission that such indemnification is against public policy as expressed in such Securities Act and is, therefore, unenforceable.
2015 Equity Incentive Plan
In 2015, the Company’s Board of Directors approved 2015 Equity Incentive Plan (“2015 Plan”), to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to employees, directors, and consultants, and to promote the success of the Company’s business. The 2015 Plan provides for the grant of stock options and restricted stock awards (“RSUs”) to purchase common stock. The Board of Directors approved 363,636 shares of Common Stock issuance under the 2015 Plan. On January 31, 2018, the Board of Directors added an additional 545,454 shares of Common Stock for issuance under the 2015 Plan. On June 15, 2021, the Board of Directors added an additional 727,272 shares of Common Stock for issuance under the 2015 Plan.
As of June 30, 2021, there were 656,666 shares available for issuance under the 2015 Plan.
Eligible recipients of option awards are employees, officers, consultants, attorneys, advisors or directors (including non-employee directors) of the Company or of any parent, subsidiary or affiliate of the Company. The Board of Directors has the authority to grant to any eligible recipient any options, restricted stock or other awards valued in whole or in part by reference to, or otherwise based on, the Company’s Common Stock; provided, however, that Incentive Options may only be granted to employees of the Company or its subsidiaries.
The provisions of each option granted need not be the same with respect to each option recipient. Option recipients have entered into award agreements with the Company, in such form as the full Board of Directors has determined.
The 2015 Plan is administered by the Board of Directors.
82
The validity of the Common Stock offered by us in this offering will be passed upon for us by Lucosky Brookman LLP, Woodbridge, New Jersey. Certain legal matters will be passed upon for the underwriter by Schiff Hardin, LLP, Washington, DC.
The financial statements of HeartBeam, Inc. as of December 31, 2020 and 2019 and for the years in the two year period ended December 31, 2020 have been included in this Registration Statement and have been so included in reliance on the report of Friedman LLP, an independent registered public accounting firm, (such report including an explanatory paragraph regarding our ability to continue as a going concern), given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
The Company files annual, quarterly and current reports, proxy statements and other information with the SEC. The Company has filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the Units being offered under this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to the Company and the securities being offered under this prospectus, please refer to the complete registration statement and the exhibits and schedules filed as a part of the registration statement.
You may read and copy the registration statement, as well as the Company’s reports, proxy statements and other information, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the Public Reference Room. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The SEC’s Internet site can be found at http://www.sec.gov. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and other reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge on the SEC’s website.
83
Financial Statements for the Years Ended
December 31, 2020 and 2019

HEARTBEAM, INC.
INDEX TO FINANCIAL STATEMENTS
|
Page |
||
|
Balance Sheets as of June 30, 2021 (unaudited) and December 31, 2020 |
F-19 |
|
|
F-20 |
||
|
F-21 |
||
|
Statements of Cash Flows for the six months ended June 30, 2021 and June 30, 2020 (unaudited) |
F-22 |
|
|
F-23 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of HeartBeam, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of HeartBeam, Inc. (the Company) as of December 31, 2020 and 2019, and the related statements of operations, stockholders’ deficit, and cash flows for each of the years in the two-year period ended December 31, 2020, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2020 in conformity with accounting principles generally accepted in the United States of America.
The Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company has recurring losses and negative cash flows from operations. As described in Note 2, these conditions, among others, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Friedman LLP
We have served as the Company’s auditor since 2020.
East Hanover, New Jersey
April 30, 2021 (except for the matter added and described in Note 1, as to which the date is October 4, 2021)
F-2
HEARTBEAM, INC.
Balance Sheets
(In thousands, except share data)
|
December 31, |
||||||||
|
2020 |
2019 |
|||||||
|
Assets |
|
|
|
|
||||
|
Current Assets: |
|
|
|
|
||||
|
Cash |
$ |
24 |
|
$ |
5 |
|
||
|
Prepaid expenses and other current assets |
|
27 |
|
|
2 |
|
||
|
Total Assets |
$ |
51 |
|
$ |
7 |
|
||
|
|
|
|
|
|||||
|
Liabilities and Stockholders’ Equity |
|
|
|
|
||||
|
Current Liabilities: |
|
|
|
|
||||
|
Accounts payable and accrued expenses (includes related party $15 and $2, respectively) |
|
489 |
|
|
312 |
|
||
|
Short-term notes |
|
— |
|
|
42 |
|
||
|
Convertible notes |
|
4,295 |
|
|
— |
|
||
|
Other – current liabilities |
|
52 |
|
|
— |
|
||
|
Total current liabilities |
|
4,836 |
|
|
354 |
|
||
|
|
|
|
|
|||||
|
Convertible notes – net of financing fees, non-current |
|
— |
|
|
3,380 |
|
||
|
|
|
|
|
|||||
|
Total Liabilities |
$ |
4,836 |
|
$ |
3,734 |
|
||
|
|
|
|
|
|||||
|
Commitments and contingencies (Note 9) |
|
|
|
|
||||
|
|
|
|
|
|||||
|
Stockholders’ Deficit |
|
|
|
|
||||
|
Common stock – $0.0001 par value; 20,000,000 shares authorized; 3,527,850 and 3,482,850 shares issued and outstanding at December 31, 2020 and 2019 |
|
— |
|
|
— |
|
||
|
Additional paid in capital |
|
11 |
|
|
1 |
|
||
|
Accumulated deficit |
|
(4,796 |
) |
|
(3,728 |
) |
||
|
Total Stockholders’ Deficit |
$ |
(4,785 |
) |
$ |
(3,727 |
) |
||
|
|
|
|
|
|||||
|
Total Liabilities and Stockholders’ Deficit |
$ |
51 |
|
$ |
7 |
|
||
See accompanying notes to the financial statements
F-3
HEARTBEAM, INC.
Statements of Operations
(In thousands, except share and per share data)
|
For the Years ended |
||||||||
|
2020 |
2019 |
|||||||
|
Operating Expenses: |
|
|
|
|
||||
|
Selling, general and administrative |
$ |
655 |
|
$ |
253 |
|
||
|
Research and development |
|
133 |
|
|
41 |
|
||
|
Total operating expenses |
|
788 |
|
|
294 |
|
||
|
|
|
|
|
|||||
|
Loss from operations |
|
(788 |
) |
|
(294 |
) |
||
|
|
|
|
|
|||||
|
Interest expense |
|
(280 |
) |
|
(242 |
) |
||
|
|
|
|
|
|||||
|
Loss before provision for income taxes |
|
(1,068 |
) |
|
(536 |
) |
||
|
|
|
|
|
|||||
|
Income tax provision |
|
— |
|
|
— |
|
||
|
|
|
|
|
|||||
|
Net Loss |
$ |
(1,068 |
) |
$ |
(536 |
) |
||
|
|
|
|
|
|||||
|
Net loss per share, basic and diluted |
$ |
(0.29 |
) |
$ |
(0.16 |
) |
||
|
|
|
|
|
|||||
|
Weighted average common shares outstanding, basic and diluted |
|
3,645,944 |
|
|
3,411,103 |
|
||
See accompanying notes to the financial statements
F-4
HEARTBEAM, INC.
Statement of Changes in Stockholders’ Deficit
(In thousands, except share data)
|
|
Additional |
Accumulated |
Total |
|||||||||||||
|
Shares |
Amount |
|||||||||||||||
|
Balance – December 31, 2018 |
3,122,653 |
$ |
— |
$ |
1 |
$ |
(3,192 |
) |
$ |
(3,191 |
) |
|||||
|
Stock issuance upon vesting of restricted stock awards |
292,091 |
|
— |
|
— |
|
— |
|
|
— |
|
|||||
|
Stock issuance upon vesting and exercise of stock options |
68,106 |
|
— |
|
— |
|
— |
|
|
— |
|
|||||
|
Net loss |
— |
|
— |
|
— |
|
(536 |
) |
|
(536 |
) |
|||||
|
Balance – December 31, 2019 |
3,482,850 |
$ |
— |
$ |
1 |
$ |
(3,728 |
) |
$ |
(3,727 |
) |
|||||
|
Stock-based compensation expense |
— |
|
— |
|
10 |
|
— |
|
|
10 |
|
|||||
|
Stock issuance upon vesting and exercise of stock options |
45,000 |
|
— |
|
— |
|
— |
|
|
— |
|
|||||
|
Net loss |
— |
|
— |
|
— |
|
(1,068 |
) |
|
(1,068 |
) |
|||||
|
Balance – December 31, 2020 |
3,527,850 |
$ |
— |
$ |
11 |
$ |
(4,796 |
) |
$ |
(4,785 |
) |
|||||
See accompanying notes to the financial statements
F-5
HEARTBEAM, INC.
Statements of Cash Flows
(In thousands)
|
For the Years ended |
||||||||
|
2020 |
2019 |
|||||||
|
Cash Flows From Operating Activities |
|
|
|
|
||||
|
Net loss |
$ |
(1,068 |
) |
$ |
(536 |
) |
||
|
Adjustments to reconcile net loss to net cash used in operating activities |
|
|
|
|
||||
|
Non-cash interest expense |
|
248 |
|
|
206 |
|
||
|
Stock-based compensation expense |
|
10 |
|
|
— |
|
||
|
Amortization of debt issuance cost |
|
28 |
|
|
36 |
|
||
|
Changes in operating assets and liabilities: |
|
|
|
|
||||
|
Prepaid expenses and other current assets |
|
(25 |
) |
|
(2 |
) |
||
|
Accounts payable and accrued expenses |
|
207 |
|
|
78 |
|
||
|
Net cash used in operating activities |
|
(600 |
) |
|
(218 |
) |
||
|
|
|
|
|
|||||
|
Cash Flows From Financing Activities |
|
|
|
|
||||
|
Proceeds from issuance of convertible notes, net of financing fees |
|
617 |
|
|
66 |
|
||
|
Proceeds from PPP loan |
|
22 |
|
|
— |
|
||
|
Proceeds from issuance of short-term notes |
|
— |
|
|
140 |
|
||
|
Repayment and interest paid on short-term loans |
|
(20 |
) |
|
— |
|
||
|
Net cash provided by financing activities |
|
619 |
|
|
206 |
|
||
|
|
|
|
|
|||||
|
Net increase (decrease) in cash |
|
19 |
|
|
(12 |
) |
||
|
Cash – Beginning |
|
5 |
|
|
17 |
|
||
|
|
|
|
|
|||||
|
Cash – Ending |
$ |
24 |
|
$ |
5 |
|
||
|
|
|
|
|
|||||
|
Supplemental Disclosures of Cash Flow Information: |
|
|
|
|
||||
|
Taxes |
$ |
— |
|
$ |
— |
|
||
|
Interest paid |
|
4 |
|
|
— |
|
||
|
|
|
|
|
|||||
|
Supplemental Disclosures of Non-cash Flow Information: |
|
|
|
|
||||
|
Conversion of short-term notes to convertible notes |
|
22 |
|
|
212 |
|
||
|
Non-cash financing fees - convertible notes |
|
— |
|
|
25 |
|
||
|
Non-cash financing fees – accounts payable |
|
— |
|
|
12 |
|
||
|
Non-cash – accounts payable converted to short term debt |
|
30 |
|
|
— |
|
||
See accompanying notes to the financial statements
F-6
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 1 — ORGANIZATION AND OPERATIONS
HeartBeam, Inc. (“HeartBeam” or the “Company”) is a development-stage company specializing in cardiovascular diagnostic technology. The Company was incorporated in 2015 as a Delaware corporation. The Company’s operations are based in Santa Clara, California and it operates in one segment.
HeartBeam’s initial focus is on timely diagnosis of a heart attack. The Company’s technology provides physicians with complete cardiac diagnostic information for a patient that is outside of a medical institution. The Electrocardiogram (“ECG”) collection device is the size of a credit card. The device sends ECG signals to the patient’s smartphone and on to a cloud-based software expert system. Results of the cloud-based analysis are presented to a qualified health care professional for immediate action including, if necessary, a telehealth visit. The Company has validated this novel technology in four clinical studies and is preparing to seek U.S. Food and Drug Administration (“FDA”) clearance of its initial products during 2021.
On September 27 , 2021, the Company filed a certificate of amendment to its amended and restated certificate of incorporation with the Secretary of State of the State of Delaware to effect a 1-for-2.75 reverse stock split of its outstanding shares of common stock. As a result of the reverse stock split, every 2.75 shares of the Company’s outstanding pre-reverse split common stock were combined and reclassified into one share of common stock. Unless otherwise noted, all share and per share data included in these financial statements retroactively reflect the 1-for-2.75 reverse stock split.
NOTE 2 — LIQUIDITY, GOING CONCERN AND OTHER UNCERTAINTIES
The Company is subject to a number of risks similar to those of early stage companies, including dependence on key individuals and products, the difficulties inherent in the development of a commercial market, the potential need to obtain additional capital, competition from larger companies, other technology companies and other technologies.
In accordance with Accounting Standards Codification (“ASC”) 205-40, Going Concern, the Company has evaluated whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these financial statements are issued. The Company has incurred losses each year since inception and has experienced negative cash flows from operations in each year since inception. As of December 31, 2020, and 2019, the Company had an accumulated deficit of approximately $4,796,000 and $3,728,000, respectively. Based on its current business plan assumptions and expected cash burn rate, the Company believes that it has insufficient cash to fund its current operations without additional financing for the next 12 months. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern.
The Company’s continued operations will depend on its ability to raise additional capital through various potential sources, such as equity and/or debt financings, or strategic relationships. Management can provide no assurance that such financing or strategic relationships will be available on acceptable terms, or at all, which would likely have a material adverse effect on the Company and its financial statements.
The financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern.
On January 30, 2020, the World Health Organization (“WHO”) announced a global health emergency, a result of the new strain of coronavirus (the “COVID-19 outbreak”) and the risks to the international community as the virus spreads globally beyond its point of origin. In March 2020, the WHO classified the COVID-19 outbreak as a pandemic, based on the rapid increase in exposure globally. Developments such as social distancing and shelter-in-place directives have impacted the Company’s operations. While the extent of this risk is still unknown, and although certain changes in telehealth benefits may be favorable to the Company, these disruptions may negatively impact the Company’s results of operations and liquidity beyond 2020.
F-7
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION
The accompanying financial statements have been prepared in conformity with US Generally Accepted Accounting Principles (“US GAAP”) and have been prepared on a basis which assumes that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business.
RECLASSIFICATION
Certain prior year amounts have been reclassified for consistency with the current presentation. These reclassifications had no effect on the reported results of operations.
USE OF ESTIMATES
The preparation of financial statements in conformity with US GAAP, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. Due to the inherent uncertainty involved in making estimates, actual results reported in future periods may be based on amounts that differ from those estimates.
CASH AND CASH EQUIVALENTS
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents. As of December 31, 2020, and 2019 there were no cash equivalents. At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation (“FDIC”). As of December 31, 2020 and 2019 there were no deposits at banks in excess of FDIC insured limits.
RESEARCH AND DEVELOPMENT EXPENSE
The Company expenses the cost of research and development as incurred. Research and development expenses consist primarily of professional services costs associated with the development of cardiovascular technologies and products.
FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company’s financial instruments consist primarily of cash, accounts payable, accrued liabilities and debt instruments.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability in the principal or most advantageous market for the asset transaction between market participants on the measurement date. Where available, fair value is based on observable market prices or is derived from such prices. The Company uses the market approach valuation technique to value its investments. The market approach uses prices and other pertinent information generated from market transactions involving identical or comparable assets or liabilities. The types of factors that the Company may consider in fair value pricing the investments include available current market data, including relevant and applicable market quotes.
Financial assets and liabilities carried at fair value are classified and disclosed in one of the following three categories:
• Level 1 — Observable inputs such as quoted prices in active markets.
• Level 2 — Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly.
• Level 3 — Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
F-8
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, the assignment of an asset or liability within the fair value hierarchy is based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability.
ACCOUNTING FOR WARRANTS
The Company classifies as equity any contracts that (i) require physical settlement or net-share settlement or (ii) gives the Company a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement). The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net-cash settle the contract if an event occurs and if that event is outside the control of the Company) or (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement).
The Company accounts for warrant instruments issued in conjunction with the Company’s common stock in permanent equity. These warrants are indexed to the Company’s stock and meet the requirements of equity classification as prescribed under ASC 815. Warrants classified as equity are initially measured at fair value, and subsequent changes in fair value are not recognized so long as the warrants continue to be classified as equity.
STOCK-BASED COMPENSATION
The Company periodically issues stock options and restricted stock awards to employees and non-employees for services. The Company has adopted ASU 2018-07 which expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. The Company accounts for such grants issued and vesting to employees and non-employees based on ASC 718, whereby the value of the award is measured on the date of grant and recognized as compensation expense over the vesting period.
The Company grants certain option holders the right to early exercise, as of December 31, 2020, 59,469 options remain unvested. Early exercised grants are not considered as an expense or included in either shares outstanding or weighted average shares outstanding until vested.
The fair value of stock options on the date of grant is calculated using the Black Scholes option pricing model, based on key assumptions such as the fair value of common stock, expected volatility and expected term. These estimates require the input of subjective assumptions, including (i) the expected stock price volatility, (ii) the calculation of the expected term of the award, (iii) the risk-free interest rate and (iv) expected dividends. These assumptions are primarily based on third-party valuations, historical data, peer company data and the judgment of management regarding future trends and other factors. The Company has estimated the expected term of its employee stock options using the “simplified” method, whereby, the expected term equals the arithmetic average of the vesting term and the original contractual term of the option due to its lack of sufficient historical data. The risk-free interest rates for periods within the expected term of the option are based on the US Treasury securities with a maturity date commensurate with the expected term of the associated award. The Company has never paid and does not expect to pay dividends in the foreseeable future. The Company accounts for forfeitures when they occur. Stock-based compensation expense recognized in the financial statements is reduced by the actual awards forfeited.
Compensation cost for restricted stock awards issued to employees and non-employees is measured using the grant date fair value of the award, and expense is recognized over the service period, adjusted to reflect actual forfeitures. There were no restricted stock awards in 2020, and all previously issued restricted stock awards were vested as of December 31, 2019.
INCOME TAXES
The Company accounts for income taxes using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis and tax carryforwards. Deferred tax assets
F-9
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
A valuation allowance is established to reduce net deferred tax assets to the amount expected to be realized The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being recognized. Changes in recognition and measurement are reflected in the period in which the change in judgment occurs. Interest and penalties related to unrecognized tax benefits are included in income tax expense.
NET LOSS PER COMMON SHARE
Basic net loss per share excludes the effect of dilution and is computed by dividing the net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding.
Diluted net loss per share is computed by giving effect to all potential shares of common stock, including stock options and warrants to the extent dilutive. Basic net loss per share was the same as diluted net loss per share for the years ended December 31, 2020 and 2019 as the inclusion of all potential common shares outstanding would have an anti-dilutive effect.
As of December 31, 2020, the penny warrants issued during 2019 have been excluded from the net loss per common share calculation following ASC 260-1-25-12A (Treatment of Contingently Issuable Shares in Weighted-Average Shares Outstanding) as they are not exercisable and while the Company is recognizing expense as it has determined that it is more likely than not that the terms of the warrant milestones will be met, (see NOTE 5), there are circumstances under which these shares would not be issued.
In accordance with ASC 260-10-45-13, exercisable penny options were included in the calculation of weighted average basic and diluted earnings per share.
The following is a summary of awards outstanding as of December 31, 2020 and 2019, which are not included in the computation of basic and diluted weighted average shares:
|
December 31, |
||||
|
2020 |
2019 |
|||
|
Stock options (excluding exercisable penny stock options) |
318,034 |
171,350 |
||
|
Warrants |
422,549 |
422,549 |
||
|
Total |
740,583 |
593,899 |
||
RECENT ACCOUNTING STANDARDS
Adopted:
In August 2018, the FASB issued ASU 2018-13, Disclosure Framework — Changes to the Disclosure Requirements for Fair Value Measurements (“ASU 2018-13”), which eliminates, adds and modifies certain disclosure requirements for fair value measurements as part of the FASB’s disclosure framework project. Adoption of this guidance is required for fiscal years and interim periods within those fiscal years, beginning after December 15, 2019. The Company adopted this guidance on January 1, 2020. The impact to the financial statements following this guidance is deemed immaterial.
Not Yet Adopted as of December 31, 2020:
In June 2016, the FASB issued ASU 2016-13 “Financial Instruments-Credit Losses-Measurement of Credit Losses on Financial Instruments”. This guidance replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable
F-10
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
information to inform credit loss estimates. The guidance applies to loans, accounts receivable, trade receivables and other financial assets measured at amortized cost, loan commitments, debt securities and beneficial interests in securitized financial assets, but the effect on the Company is projected to be limited to accounts receivable. In May 2019, the FASB issued ASU 2019-05 “Financial Instruments-Credit Losses (Topic 326)” which provides transition relief for companies adopting ASU 2016-13. This guidance amends ASU 2016-13 to allow companies to elect, upon adoption of ASU 2016-13, the fair value option on financial instruments that were previously recorded at amortized cost under certain circumstances. Companies are required to make this election on and instrument by instrument basis. The guidance will be effective for the fiscal year beginning January 1, 2023, including interim periods within that year.
In December 2019, the FASB issued ASU No. 2019-12 — Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”). ASU 2019-12 is part of the FASB’s overall simplification initiative and seeks to simplify the accounting for income taxes by updating certain guidance and removing certain exceptions. The updated guidance is effective for fiscal years beginning after December 15, 2020 and interim periods within those fiscal years. Early adoption is permitted. The Company has evaluated this guidance and has deemed the impact to be immaterial on its financial statements.
In August 2020, the FASB issued ASU No. 2020-06 (“ASU 2020-06”) “Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity.” ASU 2020-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models will result in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP. Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2020-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2020-06 will be effective January 1, 2024, for the Company. Early adoption is permitted, but no earlier than January 1, 2021, including interim periods within that year. Management is currently evaluating the effect of the adoption of ASU 2020-06 on its financial statements, but currently does not believe ASU 2020-06 will have a significant impact on the Company’s accounting.
In October 2020, the FASB issued ASU 2020-10, Codification Improvements, which updates various codification topics by clarifying or improving disclosure requirements to align with the SEC’s regulations. The Company will adopt ASU 2020-10 as of the reporting period beginning January 1, 2021. The adoption of this update is not expected to have a material effect on the Company’s financial statements.
NOTE 4 — DEBT
CONVERTIBLE NOTES
On August 21, 2015, the Board of Directors approved the 2015 Note Subscription Agreement (the “2015 Notes”) authorizing financing through the sale and issuance of 2015 convertible promissory notes (the “Financing”) for an aggregate amount not to exceed $1,000,000, with a maturity date of August, 25, 2017, which was derived from the issuance of the first 2015 Note, followed by an amendment on May 3, 2016, increasing the aggregate amount for issuance to $2,000,000 while leaving the maturity date unchanged. On March 2, 2017, a second amendment increased the aggregate amount for issuance to $2,500,000 and extended the maturity date to March 31, 2018. This was followed by the amendment dated January 18, 2018, increasing the aggregate amount for issuance to $3,500,000 and extending the maturity to December 31, 2018, followed by a fourth amendment dated September 6, 2018, increasing the aggregate amount for issuance to $4,500,000, with a maturity date of June 30, 2020, followed by a fifth amendment dated May 13, 2020, increasing the aggregate amount for issuance to $5,000,000, with a maturity date of December 31, 2021. The 2015 Notes convert to preferred stock in the event of a Qualified Financing, as defined in the 2015 Notes
F-11
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 4 — DEBT (cont.)
and occurring on or prior to the Maturity Date. In the event this occurs, the outstanding principal amount and all unpaid accrued interest shall automatically convert, under the terms of the 2015 Note, into the preferred stock issued under the Qualified Financing. At this time, the Company has not authorized any preferred stock. On March 22, 2021, the sixth amendment expanded the definition of a Qualified Financing of at least $2,000,000 as defined in the 2015 Notes to include either preferred stock or common stock. All amendments were updated in accordance with the 2015 Note Subscription Agreements and approved by the Board of Directors.
The sale and purchase of the 2015 Notes take place at closing on the date of the agreements. At closing, the Company will deliver to the investor the 2015 Note to be purchased by such investor, against receipt by the Company of the corresponding purchase price. The 2015 Notes will be registered in each investor’s name in the Company’s records. The 2015 Notes accrue interest payable at the rate of eight percent (8%) and the conversion price is equal to seventy percent (70%) of the per share price at which shares of preferred stock is to be sold.
Outstanding principal and accrued interest pursuant to the 2015 Note agreements would be accounted for as share settled debt as that would be within the scope of ASC 480, Distinguishing Liabilities from Equity. The issued debt in the 2015 Note agreements contains a qualifying feature that could trigger such settlement. The Company evaluated the qualifying feature and determined that there was less than 50% probability of triggering the qualifying feature and in accordance with the guidance, classified the 2015 Notes as liabilities.
As of December 31, 2020, the Company has $3,369,000 in 2015 Notes and $926,000 in accrued interest and as of December 31, 2019, the Company has $2,730,000 in 2015 Notes, and $678,000 in accrued interest, net of unamortized financing fees of $28,000, which are included in convertible notes — net of financing fees, in the accompanying Balance Sheets. During 2020, $22,000 of the $639,000 invested in the 2015 Notes was conversion of short-term notes and during 2019, $212,000 of the $330,000 invested in the 2015 Notes was conversion of the short-term notes.
During 2020 and 2019, the Company incurred financing fees of approximately $2,000 and $64,000, respectively. Under guidance ASC 835-30-35, costs incurred in 2019 were amortized and recognized as interest expense through June 30, 2020. The Company amortized approximately $28,000 and $36,000 for the years ended December 31, 2020 and 2019, respectively.
Included in the $66,000 financing fees is a non-cash fee of $25,000, representing an in-kind payment for services, which is included in the 2015 Notes; the remainder $41,000 reflects other fees incurred during the transaction.
SHORT TERM NOTES
On February 10, 2019, the Board of Directors approved the Bridge Financing for a principal aggregate amount of $500,000. Subject to the terms of the agreements, the purchaser agrees to lend to the Company an amount that is agreed upon, plus accrued interest (payable at the rate of eight percent 8%) on the Principal amount from the date of issuance until payment in full on maturity on August 31, 2019. The Company offered the holders of the Bridge Financing the opportunity to convert their debt into the 2015 Notes. A majority of the holders converted and the conversion closed August 31, 2019. The converted amount approximately included principal $190,000, $18,000 additional investments from holders and accrued interest $4,000 as of June 7, 2019. The Company accrued additional interest up to August 31, 2019 of approximately $4,000, which was subsequently repaid to the holders in 2020. In accordance with ASC 470-50-40-6, (modifications and exchanges), the Bridge Financing was extinguished by the issuance of the 2015 Notes.
On August 31, 2019, a $10,000 promissory note under the Bridge Financing was amended extending the maturity date to August 31, 2020. It was repaid in May 2020.
Short-term notes are included in current liabilities — short term notes, in the accompanying Balance Sheets. $42,000 in short-term notes outstanding at December 31, 2019 were settled during 2020. As settlement, the Company converted $22,000 into the 2015 Notes and the remaining $20,000, which included the amended $10,000 promissory note, a $6,000 advance and $4,000 in accrued interest, was repaid to the investors. On June 10, 2020, the Company issued a 3% interest promissory note of approximately $29,000 due on December 31, 2021 or earlier under certain
F-12
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 4 — DEBT (cont.)
events as defined in the promissory note in exchange for a vendor balance in accounts payable that was outstanding on December 31, 2019. The promissory note is outstanding at December 31, 2020 and is included in other current liabilities. During the year ended December 31, 2020, the Company incurred approximately $1,000 in interest expense.
On April 21, 2020, the Company received loan proceeds in the amount of approximately $21,000 under the Paycheck Protection Program (“PPP”), which is included in other current liabilities as of December 31, 2020. The PPP, established as part of the Coronavirus Aid, Relief and Economic Security Act (“CARES Act”), provided for loans to qualifying businesses for amounts up to 2.5 times of the average monthly payroll expenses of the qualifying business. Following the PPP guidelines, the Company filed for loan forgiveness in February 2021 and on March 4, 2021 the Small Business Administration approved the filing and cancelled the loan.
NOTE 5 — STOCKHOLDERS’ EQUITY
COMMON STOCK
During 2015 and 2016, the Company granted 3,300,363 restricted stock awards to key personnel, subject to various vesting terms. As of December 31, 2019, the awards are fully vested. Since 2016, there are no other restricted stock issuances.
During 2019, the Company issued 292,090 shares of common stock upon vesting of restricted stock awards and 68,106 shares of common stock upon exercise of vested stock options and vesting of early exercised stock options.
During 2020, the Company issued 45,000 shares of common stock upon exercise of vested stock options.
WARRANTS
In connection with the Bridge Financing, the Board of Directors approved the issuance of warrants. The Company issued 15,277 fully vested warrants as an incentive to investors with the rights to convert into a fixed number of shares of the Company’s common stock for an above market fixed price of $2.75 per share, exercisable, in whole or in part, for a period of 4 years from the date of issuance.
During 2019, milestone warrants were issued to certain executives of the Company totaling 407,272 units (“Penny Warrants”), these were valued on the date of grant at $0.0003 and will vest upon meeting certain milestones. The warrant may be exercised, in whole or in part upon the earliest to occur of: (i) following the Company’s initial public offering, the date on which the Company has a market capitalization of at least $50,000,000 for five consecutive business days; (ii) the closing of a Change of Control transaction with net proceeds to Company equity holders of at least $50,000,000; (iii) the date on which the Company receives a bona fide pre-money valuation from a third party investor of at least $50,000,000; (iv) the date on which the Holder’s continuous status as a Service Provider is terminated by the Company without Cause upon or within 12 months after a Change of Control; and (v) the date on which the Holder terminates his continuous status as a Service Provider for Good Reason within 12 months after a Change of Control.
In accordance with ASC Topic 480, Distinguishing Liabilities from Equity, as no derivative feature exists, the warrants issued to executives were classified as equity and the Company determined that as of December 31, 2020 it is not likely that these warrants would vest and as such the value of the warrants would be deemed immaterial with no impact on the accompanying financial statements.
Since these Penny Warrants have performance obligations to be met by the Company to become exercisable which are not met under any circumstance as of December 31, 2020, they are excluded from weighted-average shares outstanding in the net loss per share calculation.
F-13
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 5 — STOCKHOLDERS’ EQUITY (cont.)
There was no warrant activity during 2020. A summary of the outstanding warrants as of December 31, 2020 is as follows:
|
Number of |
Weighted |
Weighted |
|||||
|
Outstanding and exercisable – December 31, 2019 |
422,549 |
$ |
0.11 |
3.25 years |
|||
|
Outstanding and exercisable – December 31, 2020 |
422,549 |
$ |
0.11 |
2.12 years |
|||
NOTE 6 — STOCK-BASED COMPENSATION
In 2015, the Company’s Board of Directors approved the HeartBeam, Inc. 2015 Equity Incentive Plan (“2015 Plan”), to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to employees, directors, and consultants, and to promote the success of the Company’s business. The 2015 Plan provides for the grant of stock options and restricted stock awards (“RSUs”) to purchase common stock. The Board of Directors approved 363,636 shares of common stock issuance under the 2015 Plan. On January 31, 2018, the Board of Directors added an additional 545,454 shares of common stock for issuance under the 2015 Plan.
As of December 31, 2020, there were 211,212 shares available for issuance under the 2015 Plan.
STOCK OPTIONS
The following is a summary of stock option activity during the years ended December 31, 2020 and 2019:
|
Number of |
Weighted |
Average |
Aggregate |
||||||||
|
Outstanding – December 31, 2018 |
214,333 |
|
$ |
— |
8.3 |
$ |
— |
||||
|
Options granted |
168,000 |
|
|
— |
|
||||||
|
Options exercised |
(68,106 |
) |
|
— |
|
||||||
|
Options forfeited |
(16,667 |
) |
|
— |
|
||||||
|
|
|
|
|||||||||
|
Outstanding – December 31, 2019 |
297,560 |
|
$ |
— |
7.6 |
$ |
81 |
||||
|
Options granted |
214,182 |
|
$ |
0.28 |
|
||||||
|
Options exercised |
(45,000 |
) |
$ |
— |
|
||||||
|
|
|
|
|||||||||
|
Outstanding – December 31, 2020 |
466,742 |
|
$ |
0.14 |
8.2 |
$ |
81 |
||||
|
|
|
|
|||||||||
|
Exercisable – December 31, 2020 |
190,092 |
|
$ |
0.06 |
7.1 |
$ |
51 |
||||
_____________
(*) Exercise price — $0.0003 per share
(**) Intrinsic value is based on an independent appraisal of the fair market value of the Company’s common stock
F-14
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 6 — STOCK-BASED COMPENSATION (cont.)
The Company estimates the fair values of stock options using the Black-Scholes option-pricing model on the date of grant. For the years ended December 31, 2020 and 2019, the assumptions used in the Black-Scholes option pricing model, which was used to estimate the grant date fair value per option, were as follows:
|
2020 |
2019 |
|||||||
|
Weighted-average Black-Scholes option pricing model assumptions: |
|
|
|
|
||||
|
Volatility |
|
74.4 |
% |
|
63.3 |
% |
||
|
Expected term (in years) |
|
5.80 |
|
|
5.30 |
|
||
|
Risk-free rate |
|
3.2 |
% |
|
2.8 |
% |
||
|
Expected dividend yield |
|
0.0 |
% |
|
0.0 |
% |
||
|
Weighted average grant date fair value per share |
$ |
0.16 |
|
$ |
0.00 |
|
||
The following is a summary of stock-based compensation expense:
|
For the Year ended |
||||||
|
2020 |
2019 |
|||||
|
Research and development |
|
3,850 |
|
— |
||
|
Selling, general and administration |
|
5,846 |
|
— |
||
|
$ |
9,696 |
$ |
— |
|||
NOTE 7 — RELATED PARTY TRANSACTIONS
The Company’s month to month headquarter lease is in the name of the Company’s Chief Executive Officer, and the cost is reimbursed monthly.
During the course of business, the Company obtains accounting services from CTRLCFO, a firm in which an executive of the Company has significant influence, as well as Hardesty, where he is a non-managing partner. The Company incurred approximately $82,000 and $21,000 in accounting fees from these firms during the years ended December 31, 2020 and 2019, respectively. As of December 31, 2020, and 2019 the Company had balances due to these firms amounting to approximately $15,000 and $2,000, respectively.
The Company’s Directors and Officers have invested in the 2015 Notes of the Company, as have several consultants who provide services. As of December 31, 2020, and 2019, investments from Directors and Officers represents approximately $1,797,000 and $1,636,000, respectively. As December 31, 2020 and 2019, investments from consultants represents approximately $661,000 and $483,000, respectively.
NOTE 8 — INCOME TAX
Income tax expense attributable to pretax loss from continuing operations differed from the amounts computed by applying the U.S. federal income tax rate of 21% to pretax loss from continuing operations as a result of the following:
|
For the Years ended December 31, |
||||||||||||
|
2020 |
2019 |
|||||||||||
|
Computed “expected” tax benefit |
(224,100 |
) |
21.00 |
% |
(112,500 |
) |
21.00 |
% |
||||
|
Increase (reduction) in income taxes resulting from): |
|
|
|
|
||||||||
|
State tax, net of federal benefit |
(72,700 |
) |
6.77 |
% |
(37,200 |
) |
6.94 |
% |
||||
|
Permanent items |
1,700 |
|
-0.16 |
% |
800 |
|
-0.15 |
% |
||||
|
State research and development credits |
(2,500 |
) |
0.23 |
% |
(1,300 |
) |
0.24 |
% |
||||
|
Change in valuation allowance |
297,600 |
|
-27.85 |
% |
150,200 |
|
-28.03 |
% |
||||
|
|
|
|
|
|||||||||
|
Provision for taxes |
— |
|
0.00 |
% |
— |
|
0.00 |
% |
||||
F-15
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 8 — INCOME TAX (cont.)
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and liabilities are presented below as of December 31:
|
For the Years ended |
||||||||
|
2020 |
2019 |
|||||||
|
Deferred tax assets (liabilities): |
|
|
|
|
||||
|
Net operating loss carryforwards |
$ |
1,317,000 |
|
$ |
1,025,600 |
|
||
|
Research and development credits |
|
27,000 |
|
|
24,500 |
|
||
|
Other |
|
3,900 |
|
|
200 |
|
||
|
Total deferred tax assets |
|
1,347,900 |
|
|
1,050,300 |
|
||
|
Valuation Allowance |
|
(1,347,900 |
) |
|
(1,050,300 |
) |
||
|
Net Deferred Tax Assets |
|
— |
|
|
— |
|
||
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by approximately $298,000 for the period ended December 31, 2020.
As of December 31, 2020, the Company had net operating loss carryforwards for federal and state income tax purposes; each are approximately $4,700,000. If not utilized, these net federal and state operating loss carryforwards will expire beginning in 2035. The 20-year limitation was eliminated for losses generated after January 1, 2018, giving the taxpayer the ability to carry forward losses indefinitely. However, net operating losses will now be limited to 80 percent of taxable income. In assessing the realizability of the deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, net operating loss carryback potential and tax planning strategies in making these assessments.
As of December 31, 2020, the Company has federal and state tax credit carryforwards of $0 and $48,500, respectively. The state tax credit carryforwards do not expire.
Under Section 382 of the Internal Revenue Code of 1986, as amended, the Company’s ability to utilize NOLs or other tax attributes such as research tax credits, in any taxable year may be limited if the Company experiences, or has experienced, an “ownership change.” A Section 382 “ownership change” generally occurs if one or more stockholders or groups of stockholders, who own at least 5% of the Company’s stock, increase their ownership by more than 50 percentage points over their lowest ownership percentage within a rolling three-year period. Similar rules may apply under state tax laws. The Company may in the future experience, one or more Section 382 “ownership changes.” If so, the Company may not be able to utilize a material portion of its NOLs and tax credits, even if the Company achieves profitability.
The Company’s policy to recognize interest and penalties associated with unrecognized tax benefits as part of the income tax provision and include accrued interest and penalties with the related income tax liability on the Company’s balance sheet. To date, the Company has not recognized any interest and penalties in its statements of operations, nor has it accrued for or made payments for interest and penalties associated with unrecognized tax benefits.
The Company files federal and state income tax returns with varying statutes of limitations. The tax years from inception through 2020 remain open to examination due to the carryover of unused net operating losses and tax credits.
F-16
HEARTBEAM, INC.
NOTES TO FINANCIAL STATEMENTS
NOTE 9 — COMMITMENTS AND CONTINGENCIES
On May 1, 2019, the Company entered into a month to month lease agreement with Genan Family Limited Partnership, for our headquarter premises located at 2118 Walsh Ave, Suite 210, Santa Clara, CA. The agreement is for an undefined term and can be cancelled at any time, given one month’s notice by either party. The Company’s monthly rent expense associated with this agreement is approximately $1,440.
For the years ended December 31, 2020 and 2019, rent expense was approximately $17,000 and $16,000, respectively.
NOTE 10 — SUBSEQUENT EVENTS
The Company has raised an additional $315,000 in 2021 from the issuance of additional 2015 Notes.
F-17
Financial Statements for the Quarterly
Period Ended June 30, 2021

HEARTBEAM, INC.
Balance Sheets (Unaudited)
(In thousands, except share data)
|
June 30, |
December 31, |
|||||||
|
Assets |
|
|
|
|
||||
|
Current Assets: |
|
|
|
|
||||
|
Cash |
$ |
465 |
|
$ |
24 |
|
||
|
Prepaid expenses and other current assets |
|
56 |
|
|
27 |
|
||
|
Total Assets |
$ |
521 |
|
$ |
51 |
|
||
|
|
|
|
|
|||||
|
Liabilities and Stockholders’ Equity |
|
|
|
|
||||
|
Current Liabilities: |
|
|
|
|
||||
|
Accounts payable and accrued expenses (includes related party $11 and $15, respectively) |
|
561 |
|
|
489 |
|
||
|
Convertible notes, net |
|
4,194 |
|
|
4,295 |
|
||
|
Other – current liabilities |
|
30 |
|
|
52 |
|
||
|
Total current liabilities |
|
4,785 |
|
|
4,836 |
|
||
|
Total Liabilities |
$ |
4,785 |
|
$ |
4,836 |
|
||
|
Commitments and contingencies (Note 8) |
|
|
|
|
||||
|
|
|
|
|
|||||
|
Stockholders’ Deficit |
|
|
|
|
||||
|
Common stock – $0.0001 par value; 20,000,000 shares authorized; 3,547,168 and 3,527,850 shares issued and outstanding at June 30, 2021 and December 31, 2020 |
|
— |
|
|
— |
|
||
|
Additional paid in capital |
|
1,687 |
|
|
11 |
|
||
|
Accumulated deficit |
|
(5,951 |
) |
|
(4,796 |
) |
||
|
Total Stockholders’ Deficit |
$ |
(4,264 |
) |
$ |
(4,785 |
) |
||
|
Total Liabilities and Stockholders’ Deficit |
$ |
521 |
|
$ |
51 |
|
||
See accompanying notes to the unaudited financial statements
F-19
HEARTBEAM, INC.
Statements of Operations (Unaudited)
(In thousands, except share and per share data)
|
Three Months ended |
Six Months ended |
|||||||||||||||
|
2021 |
2020 |
2021 |
2020 |
|||||||||||||
|
Operating Expenses: |
|
|
|
|
|
|
|
|
||||||||
|
General and administrative |
$ |
312 |
|
$ |
133 |
|
$ |
446 |
|
$ |
227 |
|
||||
|
Research and development |
|
25 |
|
|
21 |
|
|
54 |
|
|
28 |
|
||||
|
Total operating expenses |
|
337 |
|
|
154 |
|
|
500 |
|
|
255 |
|
||||
|
Loss from operations |
|
(337 |
) |
|
(154 |
) |
|
(500 |
) |
|
(255 |
) |
||||
|
|
|
|
|
|
|
|
|
|||||||||
|
Interest expense |
|
(608 |
) |
|
(75 |
) |
|
(677 |
) |
|
(147 |
) |
||||
|
|
|
|
|
|
|
|
|
|||||||||
|
Other Income |
|
— |
|
|
— |
|
|
22 |
|
|
— |
|
||||
|
Loss before provision for income taxes |
|
(945 |
) |
|
(229 |
) |
|
(1,155 |
) |
|
(402 |
) |
||||
|
Income tax provision |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
||||
|
|
|
|
|
|
|
|
|
|||||||||
|
Net Loss |
$ |
(945 |
) |
$ |
(229 |
) |
$ |
(1,155 |
) |
$ |
(402 |
) |
||||
|
Net loss per share, basic and diluted |
$ |
(0.26 |
) |
$ |
(0.06 |
) |
$ |
(0.31 |
) |
$ |
(0.11 |
) |
||||
|
Weighted average common shares outstanding, basic and diluted |
|
3,699,762 |
|
|
3,638,315 |
|
|
3,706,550 |
|
|
3,646,159 |
|
||||
See accompanying notes to the unaudited financial statements
F-20
HEARTBEAM, INC.
Statement of Changes in Stockholders’ Deficit (Unaudited)
(In thousands, except share data)
Three Months Ended June 30, 2021 and 2020
|
|
Additional |
Accumulated Deficit |
Total |
|||||||||||||
|
Shares |
Amount |
|||||||||||||||
|
Balance – March 31, 2021 |
3,538,646 |
$ |
— |
$ |
20 |
$ |
(5,006 |
) |
$ |
(4,986 |
) |
|||||
|
Stock based compensation, |
— |
|
— |
|
23 |
|
— |
|
|
23 |
|
|||||
|
Stock issuance upon vesting and exercise of stock options |
8,522 |
|
— |
|
— |
|
— |
|
|
— |
|
|||||
|
Debt discount, share settled debt |
— |
|
— |
|
1,644 |
|
— |
|
|
1,644 |
|
|||||
|
Net loss |
— |
|
— |
|
— |
$ |
(945 |
) |
|
(945 |
) |
|||||
|
Balance – June 30, 2021 |
3,547,168 |
$ |
— |
$ |
1,687 |
$ |
(5,951 |
) |
$ |
(4,264 |
) |
|||||
|
|
|
|
|
|
|
|||||||||||
|
Balance – March 31, 2020 |
3,493,645 |
$ |
— |
$ |
1 |
$ |
(3,901 |
) |
$ |
(3,900 |
) |
|||||
|
Stock based compensation, |
— |
|
— |
|
2 |
|
— |
|
|
2 |
|
|||||
|
Stock issuance upon vesting and exercise of stock options |
10,795 |
|
— |
|
— |
|
— |
|
|
— |
|
|||||
|
Net loss |
— |
|
— |
|
— |
$ |
(229 |
) |
$ |
(229 |
) |
|||||
|
Balance – June 30, 2020 |
3,504,440 |
$ |
— |
$ |
3 |
$ |
(4,130 |
) |
$ |
(4,127 |
) |
|||||
Six Months Ended June 30, 2021 and 2020
|
|
Additional |
Accumulated |
Total |
|||||||||||||
|
Shares |
Amount |
|||||||||||||||
|
Balance – December 31, 2020 |
3,527,850 |
$ |
— |
$ |
11 |
$ |
(4,796 |
) |
$ |
(4,785 |
) |
|||||
|
Stock based compensation, |
— |
|
— |
|
32 |
|
— |
|
|
32 |
|
|||||
|
Debt discount, share settled debt |
— |
|
— |
|
1,644 |
|
— |
|
|
1,644 |
|
|||||
|
Stock issuance upon vesting and exercise of stock options |
19,318 |
|
— |
|
— |
|
— |
|
|
— |
|
|||||
|
Net loss |
— |
|
— |
|
— |
$ |
(1,155 |
) |
|
(1,155 |
) |
|||||
|
Balance – June 30, 2021 |
3,547,168 |
$ |
— |
$ |
1,687 |
$ |
(5,951 |
) |
$ |
(4,264 |
) |
|||||
|
|
|
|
|
|
|
|||||||||||
|
Balance – December 31, 2019 |
3,482,850 |
$ |
— |
$ |
1 |
$ |
(3,728 |
) |
$ |
(3,727 |
) |
|||||
|
Stock based compensation, |
— |
|
— |
|
2 |
|
— |
|
|
2 |
|
|||||
|
Stock issuance upon vesting and exercise of stock options |
21,590 |
|
— |
|
— |
|
— |
|
|
— |
|
|||||
|
Net loss |
— |
|
— |
|
— |
$ |
(402 |
) |
|
(402 |
) |
|||||
|
Balance – June 30, 2020 |
3,504,440 |
$ |
— |
$ |
3 |
$ |
(4,130 |
) |
$ |
(4,127 |
) |
|||||
See accompanying notes to the unaudited financial statements
F-21
HEARTBEAM, INC.
Statements of Cash Flows (Unaudited)
(In thousands)
|
Six Months ended |
||||||||
|
2021 |
2020 |
|||||||
|
Cash Flows From Operating Activities |
|
|
|
|
||||
|
Net loss |
$ |
(1,155 |
) |
$ |
(402 |
) |
||
|
Adjustments to reconcile net loss to net cash used in operating activities |
|
|
|
|
||||
|
Accretion expense convertible notes |
|
531 |
|
|
— |
|
||
|
Non-cash interest expense |
|
147 |
|
|
117 |
|
||
|
Stock-based compensation expense |
|
32 |
|
|
2 |
|
||
|
Amortization of debt issuance cost |
|
— |
|
|
28 |
|
||
|
PPP loan forgiveness |
|
(22 |
) |
|
— |
|
||
|
Changes in operating assets and liabilities: |
|
|
|
|
||||
|
Prepaid expenses and other current assets |
|
(29 |
) |
|
— |
|
||
|
Accounts payable and accrued expenses |
|
72 |
|
|
88 |
|
||
|
Net cash used in operating activities |
|
(424 |
) |
|
(167 |
) |
||
|
|
|
|
|
|||||
|
Cash Flows From Financing Activities |
|
|
|
|
||||
|
Proceeds from issuance of convertible notes, net of financing fees |
|
865 |
|
|
287 |
|
||
|
Proceeds from PPP & EIDL Loans |
|
— |
|
|
22 |
|
||
|
Repayment of short-term loans |
|
— |
|
|
(16 |
) |
||
|
Net cash provided by financing activities |
|
865 |
|
|
293 |
|
||
|
Net increase (decrease) in cash |
|
441 |
|
|
126 |
|
||
|
Cash – Beginning |
|
24 |
|
|
5 |
|
||
|
Cash – Ending |
$ |
465 |
|
$ |
131 |
|
||
|
Supplemental Disclosures of Cash Flow Information: |
|
|
|
|
||||
|
Taxes paid |
$ |
— |
|
$ |
— |
|
||
|
Interest paid |
|
— |
|
|
1 |
|
||
|
Supplemental Disclosures of Non-cash Flow Information: |
|
|
|
|
||||
|
Conversion of short-term notes to convertible notes |
$ |
— |
|
$ |
22 |
|
||
|
Debt discount, share settled debt |
|
1,644 |
|
|
— |
|
||
See accompanying notes to the unaudited financial statements
F-22
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 1 — ORGANIZATION AND OPERATIONS
HeartBeam, Inc. (“HeartBeam” or the “Company”) is a development-stage company specializing in cardiovascular diagnostic technology. The Company was incorporated in 2015 as a Delaware corporation. The Company’s operations are based in Santa Clara, California and it operates in one segment.
HeartBeam’s initial focus is on timely diagnosis of a heart attack. The Company’s technology provides physicians with complete cardiac diagnostic information for a patient that is outside of a medical institution. The Electrocardiogram (“ECG”) collection device is the size of a credit card. The device sends ECG signals to the patient’s smartphone and on to a cloud-based software expert system. Results of the cloud-based analysis are presented to a qualified health care professional for immediate action including, if necessary, a telehealth visit. The Company has validated this novel technology in four clinical studies and is preparing to seek U.S. Food and Drug Administration (“FDA”) clearance of its initial products late 2021 and early 2022.
On September 27, 2021, the Company filed a certificate of amendment to its amended and restated certificate of incorporation with the Secretary of State of the State of Delaware to effect a 1-for-2.75 reverse stock split of its outstanding shares of common stock. As a result of the reverse stock split, every 2.75 shares of the Company’s outstanding pre-reverse split common stock were combined and reclassified into one share of common stock. Unless otherwise noted, all share and per share data included in these unaudited financial statements retroactively reflect the 1-for-2.75 reverse stock split.
NOTE 2 — LIQUIDITY, GOING CONCERN AND OTHER UNCERTAINTIES
The Company is subject to a number of risks similar to those of early stage companies, including dependence on key individuals and products, the difficulties inherent in the development of a commercial market, the potential need to obtain additional capital, competition from larger companies, other technology companies and other technologies.
In accordance with Accounting Standards Codification (“ASC”) 205-40, Going Concern, the Company has evaluated whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these unaudited financial statements are issued. The Company has incurred losses each year since inception and has experienced negative cash flows from operations in each year since inception. As of June 30, 2021 and December 31, 2020, the Company had an accumulated deficit of approximately $5,951,000 and $4,796,000, respectively. Based on its current business plan assumptions and expected cash burn rate, the Company believes that it has insufficient cash to fund its current operations without additional financing for the next 12 months. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern.
The Company’s continued operations will depend on its ability to raise additional capital through various potential sources, such as equity and/or debt financings, or strategic relationships. Management can provide no assurance that such financing or strategic relationships will be available on acceptable terms, or at all, which would likely have a material adverse effect on the Company and its financial statements.
The unaudited financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern.
On January 30, 2020, the World Health Organization (“WHO”) announced a global health emergency, a result of the new strain of coronavirus (the “COVID-19 outbreak”) and the risks to the international community as the virus spreads globally beyond its point of origin. In March 2020, the WHO classified the COVID-19 outbreak as a pandemic, based on the rapid increase in exposure globally. Developments such as social distancing and shelter-in-place directives have impacted the Company’s operations. While the extent of this risk is still unknown, and although certain changes in telehealth benefits may be favorable to the Company, these disruptions may negatively impact the Company’s results of operations and liquidity beyond 2021.
F-23
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION
The accompanying unaudited financial statements have been prepared in conformity with US Generally Accepted Accounting Principles (“US GAAP”) and have been prepared on a basis which assumes that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business.
In the opinion of management, all adjustments (which include normal recurring adjustments) necessary to present fairly the financial position as of June 30, 2021 and December 31, 2020 and results of operations for the three and six months ended June 30, 2021 and 2020 have been made. The results of operations for the periods presented is not necessarily indicative of the results of operations expected for the full year. These unaudited financial statements should be read in conjunction with the audited financial statements for the year ended December 31, 2020.
USE OF ESTIMATES
The preparation of financial statements in conformity with US GAAP, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. Due to the inherent uncertainty involved in making estimates, actual results reported in future periods may be based on amounts that differ from those estimates.
CASH AND CASH EQUIVALENTS
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents. As of June 30, 2021 and December 31, 2020 there were no cash equivalents. At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation (“FDIC”).
RESEARCH AND DEVELOPMENT EXPENSE
The Company expenses the cost of research and development as incurred. Research and development expenses consist primarily of professional services costs associated with the development of cardiovascular technologies and products.
FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company’s financial instruments consist primarily of cash, accounts payable, accrued liabilities and debt instruments.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability in the principal or most advantageous market for the asset transaction between market participants on the measurement date. Where available, fair value is based on observable market prices or is derived from such prices. The Company uses the market approach valuation technique to value its investments. The market approach uses prices and other pertinent information generated from market transactions involving identical or comparable assets or liabilities. The types of factors that the Company may consider in fair value pricing the investments include available current market data, including relevant and applicable market quotes. Financial assets and liabilities carried at fair value are classified and disclosed in one of the following three categories:
• Level 1 — Observable inputs such as quoted prices in active markets.
• Level 2 — Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly.
• Level 3 — Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
F-24
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, the assignment of an asset or liability within the fair value hierarchy is based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability.
ACCOUNTING FOR WARRANTS
The Company classifies as equity any contracts that (i) require physical settlement or net-share settlement or (ii) gives the Company a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement). The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net-cash settle the contract if an event occurs and if that event is outside the control of the Company) or (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement).
The Company accounts for its currently issued warrant instruments in conjunction with the Company’s common stock in permanent equity. These warrants are indexed to the Company’s stock and meet the requirements of equity classification as prescribed under ASC 815. Warrants classified as equity are initially measured at fair value, and subsequent changes in fair value are not recognized so long as the warrants continue to be classified as equity.
STOCK-BASED COMPENSATION
The Company periodically issues stock options and restricted stock awards to employees and non-employees for services. The Company has adopted ASU 2018-07 which expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. The Company accounts for such grants issued and vesting to employees and non-employees based on ASC 718, whereby the value of the award is measured on the date of grant and recognized as compensation expense over the vesting period.
The Company grants certain option holders the right to early exercise, as of June 30, 2021, 40,151 options remain unvested. These early exercised grants are not considered an expense or included in either shares outstanding or weighted average shares outstanding until vested.
The fair value of stock options on the date of grant is calculated using the Black Scholes option pricing model, based on key assumptions such as the fair value of common stock, expected volatility and expected term. These estimates require the input of subjective assumptions, including (i) the expected stock price volatility, (ii) the calculation of the expected term of the award, (iii) the risk-free interest rate and (iv) expected dividends. These assumptions are primarily based on third-party valuations, historical data, peer company data and the judgment of management regarding future trends and other factors. The Company has estimated the expected term of its employee stock options using the “simplified” method, whereby, the expected term equals the arithmetic average of the vesting term and the original contractual term of the option due to its lack of sufficient historical data. The risk-free interest rates for periods within the expected term of the option are based on the US Treasury securities with a maturity date commensurate with the expected term of the associated award. The Company has never paid and does not expect to pay dividends in the foreseeable future. The Company accounts for forfeitures when they occur. Stock-based compensation expense recognized in the financial statements is reduced by the actual awards forfeited.
Compensation cost for restricted stock awards issued to employees and non-employees is measured using the grant date fair value of the award, and expense is recognized over the service period, adjusted to reflect actual forfeitures. There have been no restricted stock awards issued or outstanding as of June 30, 2021 and 2020.
INCOME TAXES
The Company accounts for income taxes using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis and tax carryforwards. Deferred tax assets
F-25
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
A valuation allowance is established to reduce net deferred tax assets to the amount expected to be realized The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being recognized. Changes in recognition and measurement are reflected in the period in which the change in judgment occurs. Interest and penalties related to unrecognized tax benefits are included in income tax expense.
NET LOSS PER COMMON SHARE
Basic net loss per share excludes the effect of dilution and is computed by dividing the net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding.
Diluted net loss per share is computed by giving effect to all potential shares of common stock, including stock options and warrants to the extent dilutive. Basic net loss per share was the same as diluted net loss per share for the three and six months ended June 30, 2021 and 2020 as the inclusion of all potential common shares outstanding would have an anti-dilutive effect.
As of June 30, 2021, the penny warrants issued during 2019 have been excluded from the net loss per common share calculation following ASC 260-1-25-12A (Treatment of Contingently Issuable Shares in Weighted-Average Shares Outstanding) as they are not exercisable and while the Company is recognizing expense as it has determined that it is more likely than not that the terms of the warrant milestones will be met, (see NOTE 5), there are circumstances under which these shares would not be issued.
In accordance with ASC 260-10-45-13, exercisable penny options were included in the calculation of weighted average basic and diluted earnings per share.
The following is a summary of awards outstanding as of June 30, 2021 and 2020, which are not included in the computation of basic and diluted weighted average shares:
|
Three Months ended |
Six Months ended |
|||||||
|
2021 |
2020 |
2021 |
2020 |
|||||
|
Stock options (excluding exercisable penny stock options) |
569,875 |
214,992 |
569,875 |
214,992 |
||||
|
Convertible debt |
2,718,023 |
— |
2,718,023 |
— |
||||
|
Warrants |
422,549 |
422,549 |
422,549 |
422,549 |
||||
|
Total |
3,710,447 |
637,541 |
3,710,447 |
637,541 |
||||
RECENT ACCOUNTING STANDARDS
Adopted:
In December 2019, the FASB issued ASU No. 2019-12 - Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”). ASU 2019-12 is part of the FASB’s overall simplification initiative and seeks to simplify the accounting for income taxes by updating certain guidance and removing certain exceptions. The updated guidance is effective for fiscal years beginning after December 15, 2020 and interim periods within those fiscal years. The Company adopted this guidance on January 1, 2021. The impact to the unaudited financial statements following this guidance is deemed immaterial.
F-26
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
In October 2020, the FASB issued ASU 2020-10, Codification Improvements, which updates various codification topics by clarifying or improving disclosure requirements to align with the SEC’s regulations. The Company adopted ASU 2020-10 as of January 1, 2021. The impact to the unaudited financial statements following this guidance is deemed immaterial.
Not Yet Adopted as of June 30, 2021:
In June 2016, the FASB issued ASU 2016-13 “Financial Instruments-Credit Losses-Measurement of Credit Losses on Financial Instruments”. This guidance replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. The guidance applies to loans, accounts receivable, trade receivables and other financial assets measured at amortized cost, loan commitments, debt securities and beneficial interests in securitized financial assets, but the effect on the Company is projected to be limited to accounts receivable. In May 2019, the FASB issued ASU 2019-05 “Financial Instruments-Credit Losses (Topic 326)” which provides transition relief for companies adopting ASU 2016-13. This guidance amends ASU 2016-13 to allow companies to elect, upon adoption of ASU 2016-13, the fair value option on financial instruments that were previously recorded at amortized cost under certain circumstances. Companies are required to make this election on and instrument by instrument basis. The guidance will be effective for the fiscal year beginning January 1, 2023, including interim periods within that year.
In August 2020, the FASB issued ASU No. 2020-06 (“ASU 2020-06”) “Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity.” ASU 2020-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models will result in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP. Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2020-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2020-06 will be effective January 1, 2024, for the Company. Early adoption is permitted, but no earlier than January 1, 2021, including interim periods within that year. Management has not early adopted ASU 2020-06 and is currently evaluating the effects of the adoption, but currently believes the guidance will not have a significant impact on the Company’s accounting.
NOTE 4 — DEBT
CONVERTIBLE NOTES
On August 21, 2015, the Board of Directors approved the 2015 Note Subscription Agreement (the “2015 Notes”) authorizing financing through the sale and issuance of 2015 convertible promissory notes (the “Financing”) for an aggregate amount not to exceed $1,000,000, with a maturity date of August, 25, 2017, which was derived from the issuance of the first 2015 Note, followed by an amendment on May 3, 2016, increasing the aggregate amount for issuance to $2,000,000 while leaving the maturity date unchanged. On March 2, 2017, a second amendment increased the aggregate amount for issuance to $2,500,000 and extended the maturity date to March 31, 2018. This was followed by the amendment dated January 18, 2018, increasing the aggregate amount for issuance to $3,500,000 and extending the maturity to December 31, 2018, followed by a fourth amendment dated September 6, 2018, increasing the aggregate amount for issuance to $4,500,000, with a maturity date of June 30, 2020, followed by a fifth amendment dated May 13, 2020, increasing the aggregate amount for issuance to $5,000,000, with a maturity date of December 31,
F-27
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 4 — DEBT (cont.)
2021. The 2015 Notes convert to preferred stock in the event of a Qualified Financing, as defined in the 2015 Notes and occurring on or prior to the Maturity Date. In the event this occurs, the outstanding principal amount and all unpaid accrued interest shall automatically convert, under the terms of the 2015 Note, into the preferred stock issued under the Qualified Financing. At this time, the Company has not authorized any preferred stock. On March 22, 2021, the sixth amendment expanded the definition of a Qualified Financing of at least $2,000,000 as defined in the 2015 Notes to include either preferred stock or common stock. All amendments were updated in accordance with the 2015 Note Subscription Agreements and approved by the Board of Directors. The Company has accounted for the sixth amendment to the 2015 Notes in accordance with ASC 470-50-40-6, (modifications and exchanges), under modification accounting and there was no impact to the financial statements as a result of the amendment to the 2015 Notes.
The sale and purchase of the 2015 Notes take place at closing on the date of the agreements. At closing, the Company will deliver to the investor the 2015 Note to be purchased by such investor, against receipt by the Company of the corresponding purchase price. The 2015 Notes will be registered in each investor’s name in the Company’s records. The 2015 Notes accrue interest payable at the rate of eight percent (8%) and the conversion price is equal to seventy percent (70%) of the per share price at which shares of preferred stock or common stock is to be sold.
During the quarter ended June 30, 2021, the Company’s assessment was that the conversion of the 2015 Notes prior to maturity in a “Qualified Financing” was the predominant feature, and the 2015 Notes would be share-settled debt at 30% discount, and as of March 31, 2021, there was less than 50% probability that a Qualified Financing would occur, therefore, the Company elected not to record any discounts since the cash repayment of principal and accrued interest at maturity was the most probable outcome.
During the three months ended June 30, 2021, the Company revised its assessment of the probability of a Qualified Financing occurring before maturity of the 2015 Notes to be greater than 50% (more likely than not). Under guidance ASC 480, the Company recorded the amount of the 2015 Notes’ 30% conversion discount of the sum of principle and accrued interest to the earliest of conversion date (if known) or maturity. The Company recorded approximately $1,644,000 as debt discount and accreted approximately $531,000 as interest expense during the three and six months ended June 30, 2021.
As of June 30, 2021, the Company has $4,234,000 in 2015 Notes and $1,073,000 in accrued interest, offset by $1,113,000 debt discount and as of December 31, 2020, the Company has $3,369,000 in 2015 Notes, and $926,000 in accrued interest.
In 2019, the Company incurred financing fees of approximately $64,000. Under guidance ASC 835-30-35, these costs have been amortized and recognized as interest expense over the life of the 2015 Notes. During the three and six months ended June 30, 2020, the Company amortized approximately $14,000 and $28,000 of the remaining balance as interest expense. There was no such expense during the three and six months ended June 30, 2021.
SHORT TERM LOANS
On April 21, 2020, the Company received loan proceeds in the amount of approximately $22,000 under the Paycheck Protection Program (“PPP”), which was included in other current liabilities as of December 31, 2020. The PPP, established as part of the Coronavirus Aid, Relief and Economic Security Act (“CARES Act”), provided for loans to qualifying businesses for amounts up to 2.5 times of the average monthly payroll expenses of the qualifying business. Following the PPP guidelines, the Company filed for loan forgiveness in February 2021 and on March 4, 2021 the Small Business Administration approved the filing and forgave the loan. The Company recognized the gain on forgiveness in other income on the statements of operations during the six months ended June 30, 2021.
F-28
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 5 — STOCKHOLDERS’ EQUITY
COMMON STOCK
During the six months ended June 30, 2021 and 2020 the Company issued 19,318 and 21,590 shares of common stock upon exercise of vested stock options.
WARRANTS
In connection with the short term notes issued in 2019, the Board of Directors approved the issuance of warrants. The Company issued 15,277 fully vested warrants as an incentive to investors with the rights to convert into a fixed number of shares of the Company’s common stock for an above market fixed price of $2.75 per share, exercisable, in whole or in part, for a period of 4 years from the date of issuance.
During 2019, milestone warrants were issued to certain executives of the Company totaling 407,272 units (“Penny Warrants”), these were valued on the date of grant at $0.0003 and will vest upon meeting certain milestones. The warrant may be exercised, in whole or in part upon the earliest to occur of: (i) following the Company’s initial public offering, the date on which the Company has a market capitalization of at least $50,000,000 for five consecutive business days; (ii) the closing of a Change of Control transaction with net proceeds to Company equity holders of at least $50,000,000; (iii) the date on which the Company receives a bona fide pre-money valuation from a third party investor of at least $50,000,000; (iv) the date on which the Holder’s continuous status as a Service Provider is terminated by the Company without Cause upon or within 12 months after a Change of Control; and (v) the date on which the Holder terminates his continuous status as a Service Provider for Good Reason within 12 months after a Change of Control.
In accordance with ASC Topic 480, Distinguishing Liabilities from Equity, as no derivative feature exists, the warrants issued to executives were classified as equity and the Company determined that as of June 30, 2021 and December 31, 2020 it is not likely that these warrants would vest and as such the value of the warrants would be deemed immaterial with no impact on the accompanying unaudited financial statements.
Since these Penny Warrants have performance obligations to be met by the Company to become exercisable which are not met under any circumstance as of June 30, 2021, they are excluded from weighted-average shares outstanding in the net loss per share calculation.
There was no warrant activity during the six months ended June 30, 2021 and 2020. A summary of the outstanding warrants as of June 30, 2021 is as follows:
|
Number of |
Weighted |
Weighted |
|||||
|
Outstanding and exercisable – December 31, 2020 |
422,549 |
$ |
0.11 |
2.12 years |
|||
|
Outstanding and exercisable – June 30, 2021 |
422,549 |
$ |
0.11 |
1.62 years |
|||
NOTE 6 — STOCK-BASED COMPENSATION
In 2015, the Company’s Board of Directors approved the HeartBeam, Inc. 2015 Equity Incentive Plan (“2015 Plan”), to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to employees, directors, and consultants, and to promote the success of the Company’s business. The 2015 Plan provides for the grant of stock options and restricted stock awards (“RSUs”) to purchase common stock. The Board of Directors approved 363,636 shares of common stock issuance under the 2015 Plan. On January 31, 2018, the Board of Directors added an additional 545,454 shares of common stock for issuance under the 2015 Plan. On June 15, 2021, the Board of Directors added an additional 727,272 shares of common stock for issuance under the 2015 Plan.
As of June 30, 2021, there were 656,666 shares available for issuance under the 2015 Plan.
F-29
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 6 — STOCK-BASED COMPENSATION (cont.)
STOCK OPTIONS
The following is a summary of stock option activity during the six months ended June 30, 2021:
|
Number of |
Weighted |
Average |
Aggregate |
||||||||
|
Outstanding – December 31, 2020 |
466,742 |
|
$ |
0.14 |
8.2 |
$ |
81 |
||||
|
Options granted |
285,454 |
|
$ |
2.17 |
|
||||||
|
Forfeitures |
(3,636 |
) |
$ |
— |
|
||||||
|
Options exercised |
(19,318 |
) |
$ |
— |
|
||||||
|
Outstanding – June 30, 2021 |
729,242 |
|
$ |
0.94 |
$ |
1,367 |
|||||
|
Exercisable – June 30, 2021 |
243,426 |
|
$ |
0.14 |
$ |
648 |
|||||
____________
(*) $ — Indicates exercise price $0.000275 per share
(**) Intrinsic value is based on an independent appraisal of the fair market value of the Company’s common stock.
The Company estimates the fair values of stock options using the Black-Scholes option-pricing model on the date of grant. For the six months ended June 30, 2021 and 2020, the assumptions used in the Black-Scholes option pricing model, which was used to estimate the grant date fair value per option, were as follows:
|
Six Months ended |
||||||
|
2021 |
2020 |
|||||
|
Weighted-average Black-Scholes option pricing model assumptions: |
|
|
||||
|
Volatility |
76.8% – 76.9% |
|
74.61 |
% |
||
|
Expected term (in years) |
5.66 |
|
5.87 |
|
||
|
Risk-free rate |
0.48% – 0.82% |
|
0.39 |
% |
||
|
Expected dividend yield |
0.0% |
|
0 |
% |
||
|
Weighted average grant date fair value per option |
$ 1.59 – 1.81 |
$ |
0.16 |
|
||
The following is a summary of stock-based compensation expense:
|
Three Months ended |
Six Months ended |
|||||||||||
|
2021 |
2020 |
2021 |
2020 |
|||||||||
|
Research and development |
|
2,445 |
|
— |
|
6,440 |
|
— |
||||
|
General and administration |
|
20,959 |
|
2,199 |
|
26,048 |
|
2,617 |
||||
|
$ |
23,404 |
$ |
2,199 |
$ |
32,488 |
$ |
2,617 |
|||||
NOTE 7 — RELATED PARTY TRANSACTIONS
The Company’s month to month headquarters lease is in the name of the Company’s Chief Executive Officer, and the cost is reimbursed monthly.
During the course of business, the Company obtains accounting services from CTRLCFO, a firm in which an executive of the Company has significant influence, as well as Hardesty, where he is a non-managing partner. The Company incurred accounting fees from these firms of approximately $32,000 and $55,000 during the three and six months ended June 30, 2021, respectively, and $5,000 and $8,000 during the three and six months ended June 30, 2020, respectively. As of June 30, 2021 and December 31, 2020, the Company had balances due to these firms amounting to approximately $11,000 and $15,000, respectively.
F-30
HEARTBEAM, INC.
NOTES TO UNAUDITED FINANCIAL STATEMENTS
NOTE 7 — RELATED PARTY TRANSACTIONS (cont.)
The Company’s Directors and Officers have invested in the 2015 Notes of the Company, as have several consultants who provide services. As of June 30, 2021, and December 31, 2020, investments from Directors and Officers represents approximately $2,308,000 and $1,797,000, respectively. As of June 30, 2021 and December 31, 2020, investments from consultants represents approximately $886,000 and $661,000, respectively.
NOTE 8 — COMMITMENTS AND CONTINGENCIES
On May 1, 2019, the Company entered into a month to month lease agreement with Genan Family Limited Partnership, for our headquarters premises located at 2118 Walsh Ave, Suite 210, Santa Clara, CA. The agreement is for an undefined term and can be cancelled at any time, given one month’s notice by either party. The Company’s monthly rent expense associated with this agreement is approximately $1,440.
For the three and six months ended June 30, 2021 and 2020, rent expense was approximately $4,000 and $8,000, respectively.
F-31
Units

HEARTBEAM, INC.
_____________________
PROSPECTUS
_____________________
Book-Running Manager
THE BENCHMARK COMPANY
Until , 2021 (25 days after the date of this prospectus), all dealers that buy, sell or trade these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to its unsold allotments or subscriptions.
, 2021
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth the expenses in connection with this registration statement. All of such expenses are estimates, other than the filing fees payable to the Securities and Exchange Commission and to FINRA.
|
Amount |
|||
|
SEC registration fee |
$ |
4,000 |
|
|
FINRA filing fee |
$ |
20,000 |
|
|
The Nasdaq Capital Market initial listing fee |
$ |
50,000 |
|
|
Accounting fees and expenses |
$ |
76,000 |
|
|
Legal fees and expenses |
$ |
265,000 |
|
|
Printing and engraving expenses |
$ |
60,000 |
|
|
Miscellaneous |
$ |
175,000 |
|
|
Total |
$ |
650,000 |
|
All amounts are estimated except the SEC registration fee, the FINRA filing fee, and The Nasdaq Capital Market initial listing fee.
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Our Articles of Incorporation and our Bylaws provide for indemnification of our directors and officers. Our Bylaws provide that we will indemnify any person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative (other than an action by or in the right of the corporation) by reason of the fact that such person is or was a director or officer of the corporation, against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the Company, and with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere or its equivalent will not, without more, create a presumption that the person did not act in good faith and in a manner which such person reasonably believed to be in or not opposed to the best interest of the corporation, and, with respect to any criminal action or proceeding, had reasonable caused to believe that his conduct was unlawful. The Company may by action of its Board of Directors, grant rights to indemnification and advancement of expenses to employees and agents of the Company with the same scope and effects as the indemnification provisions for officers and directors.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
On August 21, 2015, our Board of Directors approved the 2015 Note Subscription Agreement (the “2015 Notes”) authorizing financing through the sale and issuance of 2015 convertible promissory notes (the “Financing”). These are the only securities not registered under the Securities Act. These 2015 Notes accrue interest payable at the rate of eight percent (8%) and the conversion price is equal to seventy percent (70%) of the per share price at which shares of stock is to be sold in a qualifying financing, which would include an Initial Public Offering under this Prospectus. They otherwise mature December 31, 2021.
Sales of these securities for the last three years have been to three groups, our directors and officers, consultants and other accredited investors.
|
Timeframe |
Directors and Officers |
Consultants |
Other Accredited |
||||||
|
August – December 2018 |
$ |
— |
$ |
— |
$ |
— |
|||
|
2019 |
$ |
175,000 |
$ |
58,000 |
$ |
97,000 |
|||
|
2020 |
$ |
52,000 |
$ |
140,000 |
$ |
447,000 |
|||
|
2021 to the date of this Prospectus |
$ |
550,000 |
$ |
400,000 |
$ |
765,000 |
|||
II-1
In connection with short term notes issued in 2019, we issued 15,277 fully vested warrants. The warrants convert into a fixed number of shares of our common stock at $2.75 per share, exercisable, in whole or in part, for a period of 4 years from the date of issuance. None have been exercised to date.
Issuance of these warrants have been to three groups, our directors and officers, consultants and other accredited investors.
|
Timeframe |
Directors |
Consultants |
Other Accredited Investors |
|||
|
2019 |
10,912 |
3,637 |
728 |
On February 12, 2019, we issued 407,272 milestone warrants to two of our Directors and Officers. These Penny Warrants were valued on the date of grant at $0.0003 and vest upon meeting certain milestones as defined in the agreements, which would include an Initial Public Offering under this Prospectus and the date on which the Company has a market capitalization of at least $50,000,000 for five consecutive business days.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
____________
* Previously filed.
** Filed herewith
+ To be filed by amendment.
II-2
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
II-3
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Santa Clara, State of California, on the 1st day of November 2021.
|
HEARTBEAM, INC. |
||||
|
By: |
/s/ Branislav Vajdic |
|||
|
Name: |
Branislav Vajdic |
|||
|
Title: |
Chief Executive Officer |
|||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
Title |
Date |
||
|
/s/ Branislav Vajdic |
Chief Executive Officer and Director |
November 1, 2021 |
||
|
Branislav Vajdic |
(Principal Executive Officer) |
|||
|
* |
Chief Financial Officer |
November 1, 2021 |
||
|
Richard Brounstein |
(Principal Financial and Accounting Officer) |
|||
|
* |
Executive Chairman of the Board of Directors |
November 1, 2021 |
||
|
Richard Ferrari |
||||
|
* |
Director |
November 1, 2021 |
||
|
Willem Elfrink |
||||
|
* |
Director |
November 1, 2021 |
||
|
Marga Ortigas-Wedekind |
||||
|
* |
Director |
November 1, 2021 |
||
|
George de Urioste |
|
*/s/ Branislav Vajdic |
||
|
Branislav Vajdic |
||
|
Attorney-in Fact |
II-5