
Medical Grade Heart Attack Detection Always with the patient Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration no. 333 - 259358 Relating to Preliminary Prospectus dated October 12, 2021

This presentation includes forward - looking statements that involve substantial risks and uncertainties . All statements, other than statements of historical facts, included in this presentation regarding forward - looking statements . The words “believe”, “anticipate”, “intend”, “expect”, “target”, “goal”, “estimate”, “plan”, “assume”, “may”, “will”, “predict”, “project”, “would”, “could” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words . We may not actually achieve the plans, intentions or expectations disclosed in our forward - looking statements, and you should not place undue reliance on our forward - looking statements . We have based these forward - looking statements on our current expectations and projections about future events, nevertheless, actual results or events could differ materially from the plans, intentions and expectations disclosed in, or implied by, the forward - looking statements we make . Factors that could cause such differences, but are not limited to, are our strategy, future operations, regulatory process, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth . MARKET, INDUSTRY AND OTHER DATA This presentation includes market and industry data and forecasts that the Company has developed from independent research reports, publicly available information, various industry publications, other published industry sources or the Company’s internal data and estimates . Independent research reports, industry publications and other published industry sources generally indicate that the information contained therein was obtained from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information . Although the Company believes that the publications and reports are reliable, the Company has not independently verified the data and makes no representation or warranty with respect to the accuracy of such information . Any and all trademarks and trade names referred to in this presentation are the property of their respective owners . The Company’s internal data, estimates and forecasts are based on information obtained from trade and business organizations and other contracts in the markets in which it operates and management’s understanding of industry conditions . Although the Company believes that such information is reliable, the Company has not had such information verified by any independent sources . DISCLAIMERS 2

STATEMENT ABOUT FREE WRITING PROSPECTUS This free writing prospectus relates to the proposed initial public offering of common stock of HeartBeam, Inc., which are be ing registered on a registration statement on Form S - 1 (File No. 333 - 259358) (the “Registration Statement”). This free writing prospectus should be read together with the preliminary prospectus dated October 12, 2021, included in the Registration Statement (the “Preliminary Prospectus”), which can be accessed through the following link: https://www.sec.gov/cgi - bin/browse - edgar?company=Heartbeam%2C+Inc.&match=&filenum=&State=CA&Country=&SIC=&myowner=exclude&action=getcompany We have filed a Registration Statement, including the Preliminary Prospectus, with the SEC with respect to the offering of ou r securities to which this communication relates. The Registration Statement has not yet become effective. Before you invest, y ou should read the Preliminary Prospectus (including the risk factors described therein) and, when available, the final prospect us relating to the offering, and the other documents filed with the SEC, for more complete information about us and the offering . Y ou may obtain these documents, including the Preliminary Prospectus, for free by visiting EDGAR on the SEC website at http://www.sec.gov. Alternatively, the company or the underwriter for the offering will arrange to send you the Preliminary Prospectus and, when available, the final prospectus and/or any supplements thereto, if you contact The Benchmark Company, LLC Attention: Prospectus Department, 150 E. 58 th Street, 17 th Floor, New York, New York 10155, by e - mail at prospectus@benchmarkcompany.com or by telephone at (212) 312 - 6700. 3
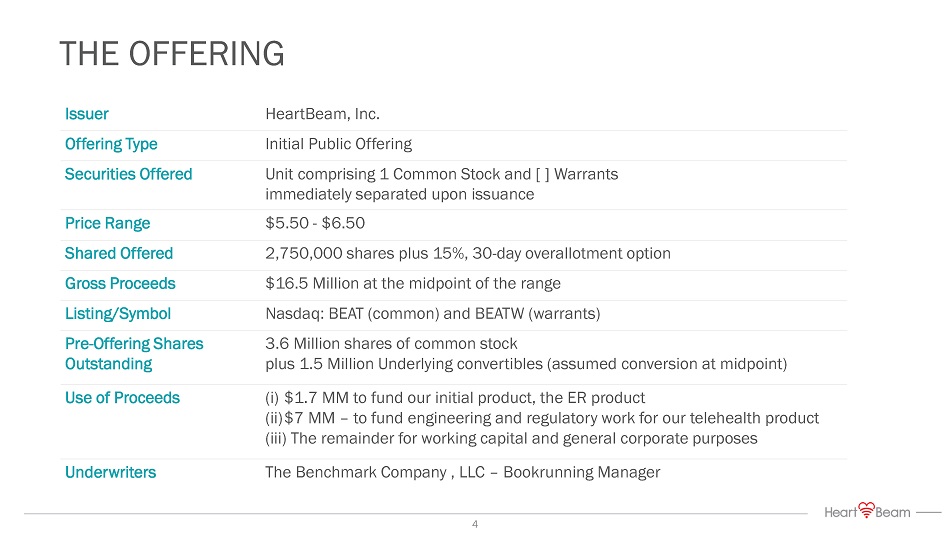
THE OFFERING Issuer HeartBeam, Inc. Offering Type Initial Public Offering Securities Offered Unit comprising 1 Common Stock and [ ] Warrants immediately separated upon issuance Price Range $5.50 - $6.50 Shared Offered 2,750,000 shares plus 15%, 30 - day overallotment option Gross Proceeds $16.5 Million at the midpoint of the range Listing/Symbol Nasdaq: BEAT (common) and BEATW (warrants) Pre - Offering Shares Outstanding 3.6 Million shares of common stock plus 1.5 Million Underlying convertibles (assumed conversion at midpoint) Use of Proceeds (i) $1.7 MM to fund our initial product, the ER product (ii) $7 MM – to fund engineering and regulatory work for our telehealth product (iii) The remainder for working capital and general corporate purposes Underwriters The Benchmark Company , LLC – Bookrunning Manager 4

ABOUT US RICH FERRARI Over 40 years of experience in Medical Device Start - ups Co - founder of De Novo Ventures which has $650M under management Executive Chairman BRANISLAV VAJDIC Founder of HeartBeam Over 30 years of experience in technology development and senior management positions Chief Executive Officer 30 years of experience in health technology senior management Extensive public and private company CFO experience Chief Financial Officer RICK BROUNSTEIN Founded in 2015, located in Santa Clara, CA Remote heart attack detection: huge, well known and previously unsolved problem with a massive worldwide market. HeartBeam has developed a system used by patients at home to help their physicians assess whether chest pain is the result of a heart attack (MI). Over 35 years of experience in the cardiovascular medical device industry Last 23 years in medical device startups with 4 exits EVP, Chief Business Officer JON HUNT HeartBeam Inc . ABOUT US 5
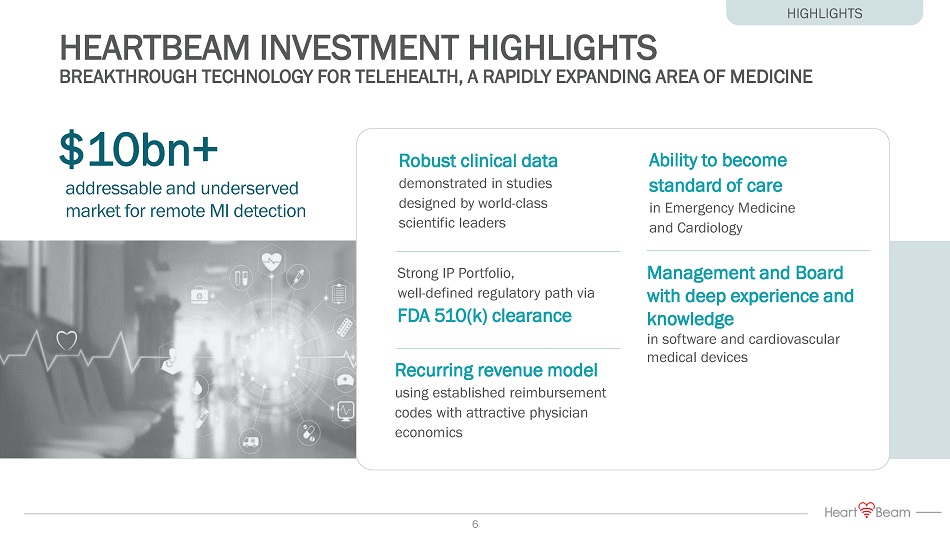
HEARTBEAM INVESTMENT HIGHLIGHTS BREAKTHROUGH TECHNOLOGY FOR TELEHEALTH, A RAPIDLY EXPANDING AREA OF MEDICINE $10bn+ Ability to become standard of care in Emergency Medicine and Cardiology Robust clinical data demonstrated in studies designed by world - class scientific leaders Strong IP Portfolio, well - defined regulatory path via FDA 510(k) clearance Recurring revenue model using established reimbursement codes with attractive physician economics Management and Board with deep experience and knowledge in software and cardiovascular medical devices addressable and underserved market for remote MI detection HIGHLIGHTS 6
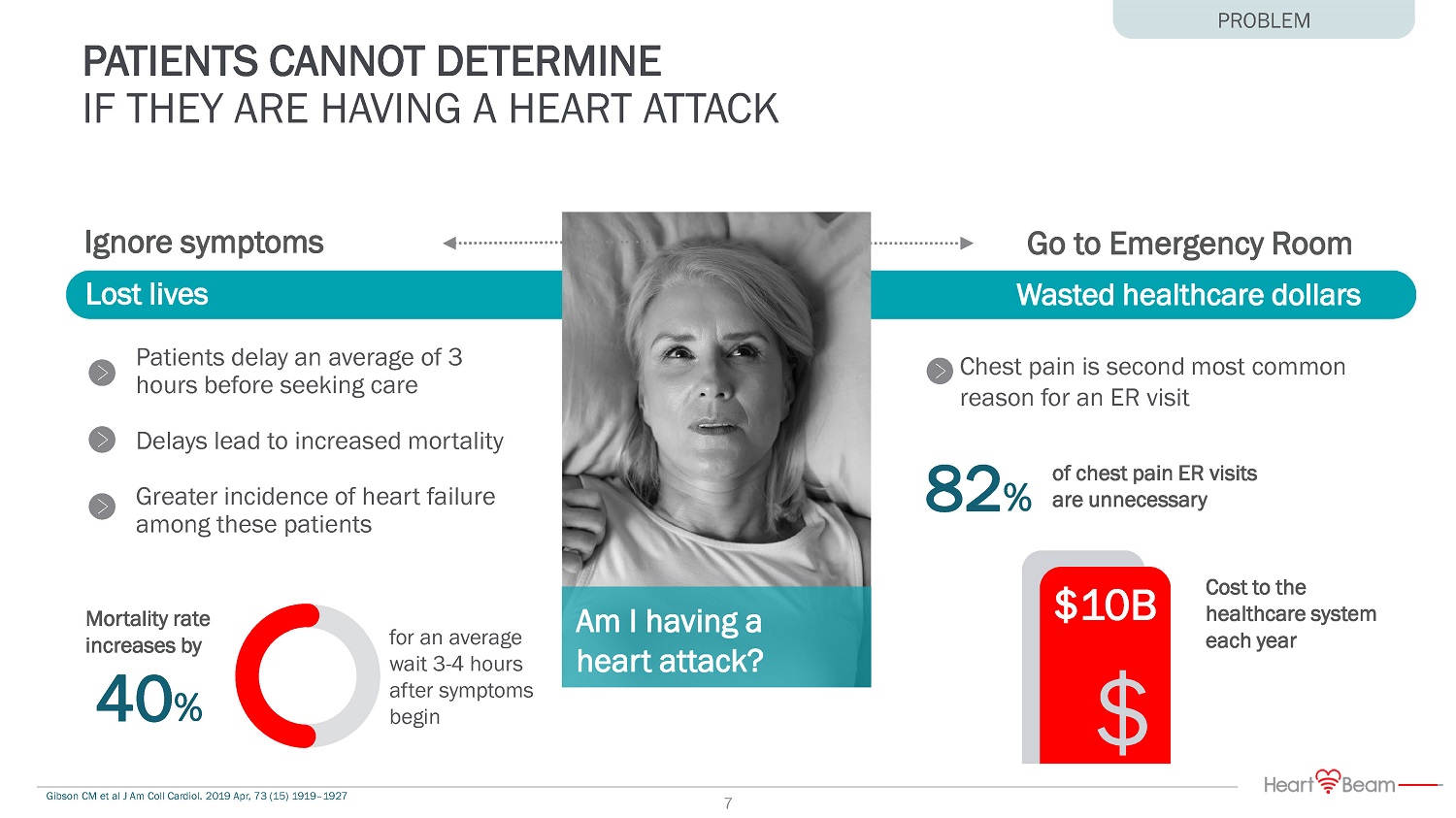
82 % Cost to the healthcare system each year $10B $ Patients delay an average of 3 hours before seeking care Delays lead to increased mortality Greater incidence of heart failure among these patients for an average wait 3 - 4 hours after symptoms begin Mortality rate increases by 40 % Wasted healthcare dollars Chest pain is second most common reason for an ER visit of chest pain ER visits are unnecessary Go to Emergency Room Ignore symptoms Lost lives Am I having a heart attack? Gibson CM et al J Am Coll Cardiol. 2019 Apr, 73 (15) 1919 – 1927 PATIENTS CANNOT DETERMINE IF THEY ARE HAVING A HEART ATTACK PROBLEM 7
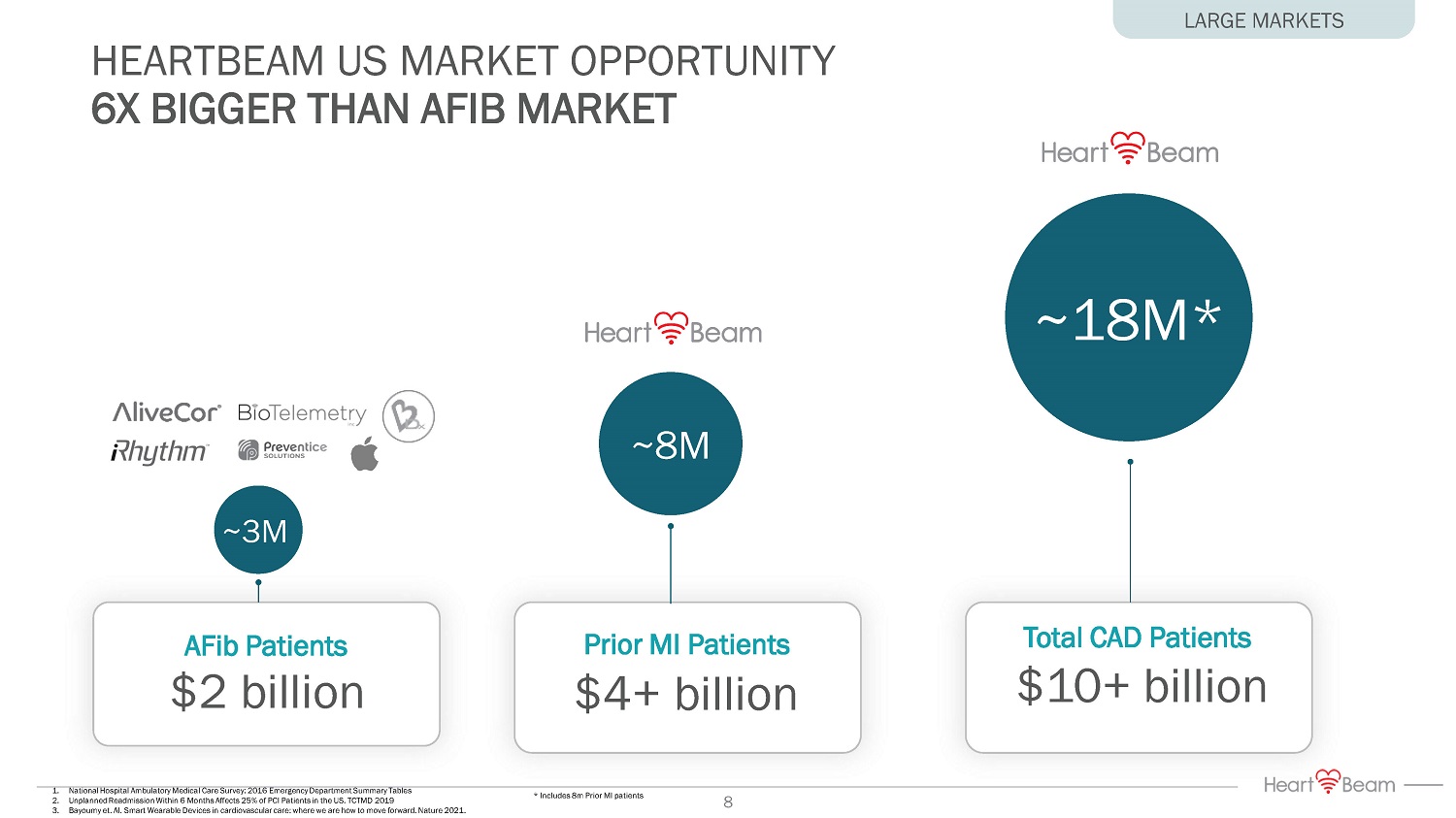
AFib Patients Prior MI Patients $4+ billion 1. National Hospital Ambulatory Medical Care Survey: 2016 Emergency Department Summary Tables 2. Unplanned Readmission Within 6 Months Affects 25% of PCI Patients in the US. TCTMD 2019 3. Bayoumy et. Al. Smart Wearable Devices in cardiovascular care: where we are how to move forward. Nature 2021. * Includes 8m Prior MI patients $2 billion ~3M HEARTBEAM US MARKET OPPORTUNITY 6X BIGGER THAN AFIB MARKET LARGE MARKETS Total CAD Patients $10+ billion ~8M ~18M* 8
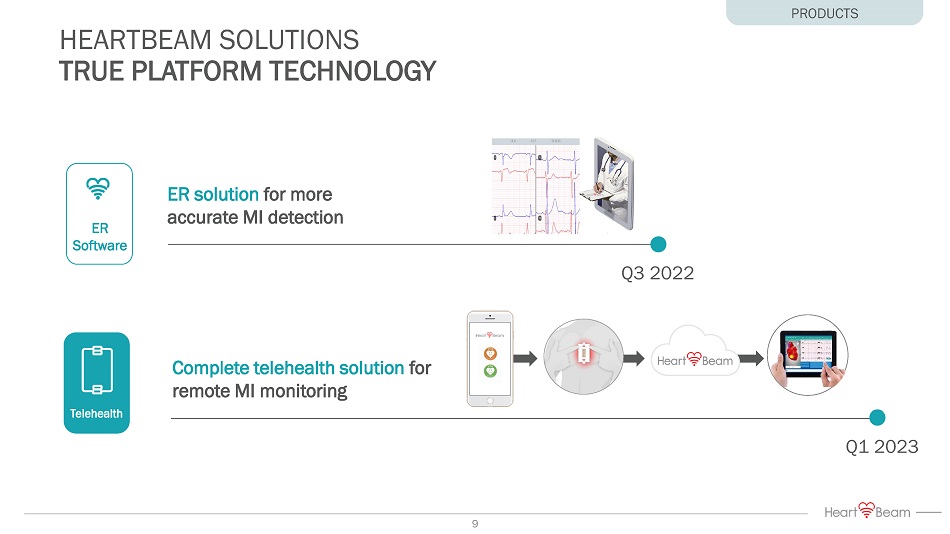
ER Software Complete telehealth solution for remote MI monitoring ER solution for more accurate MI detection Q3 2022 Q1 2023 HEARTBEAM SOLUTIONS TRUE PLATFORM TECHNOLOGY PRODUCTS Telehealth 9
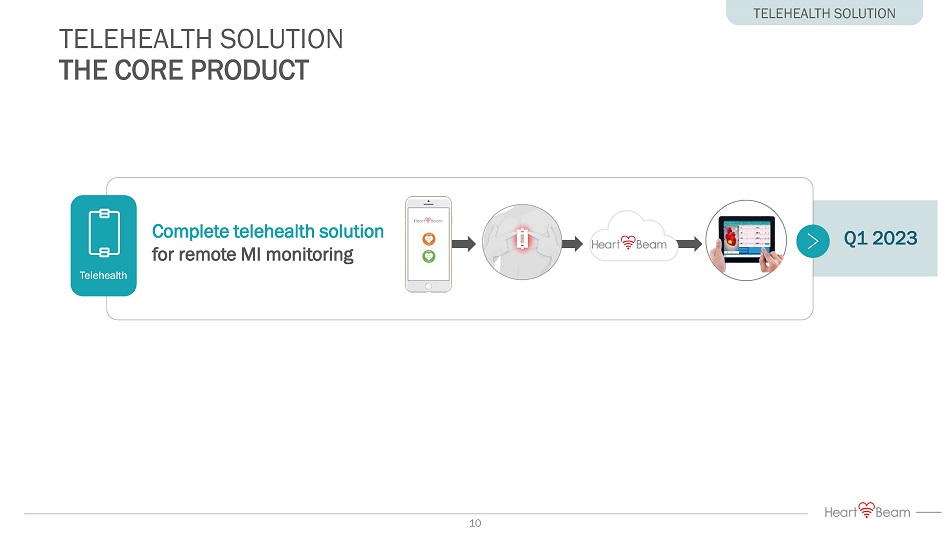
TELEHEALTH SOLUTION THE CORE PRODUCT TELEHEALTH SOLUTION Q1 2023 Telehealth Complete telehealth solution for remote MI monitoring 10
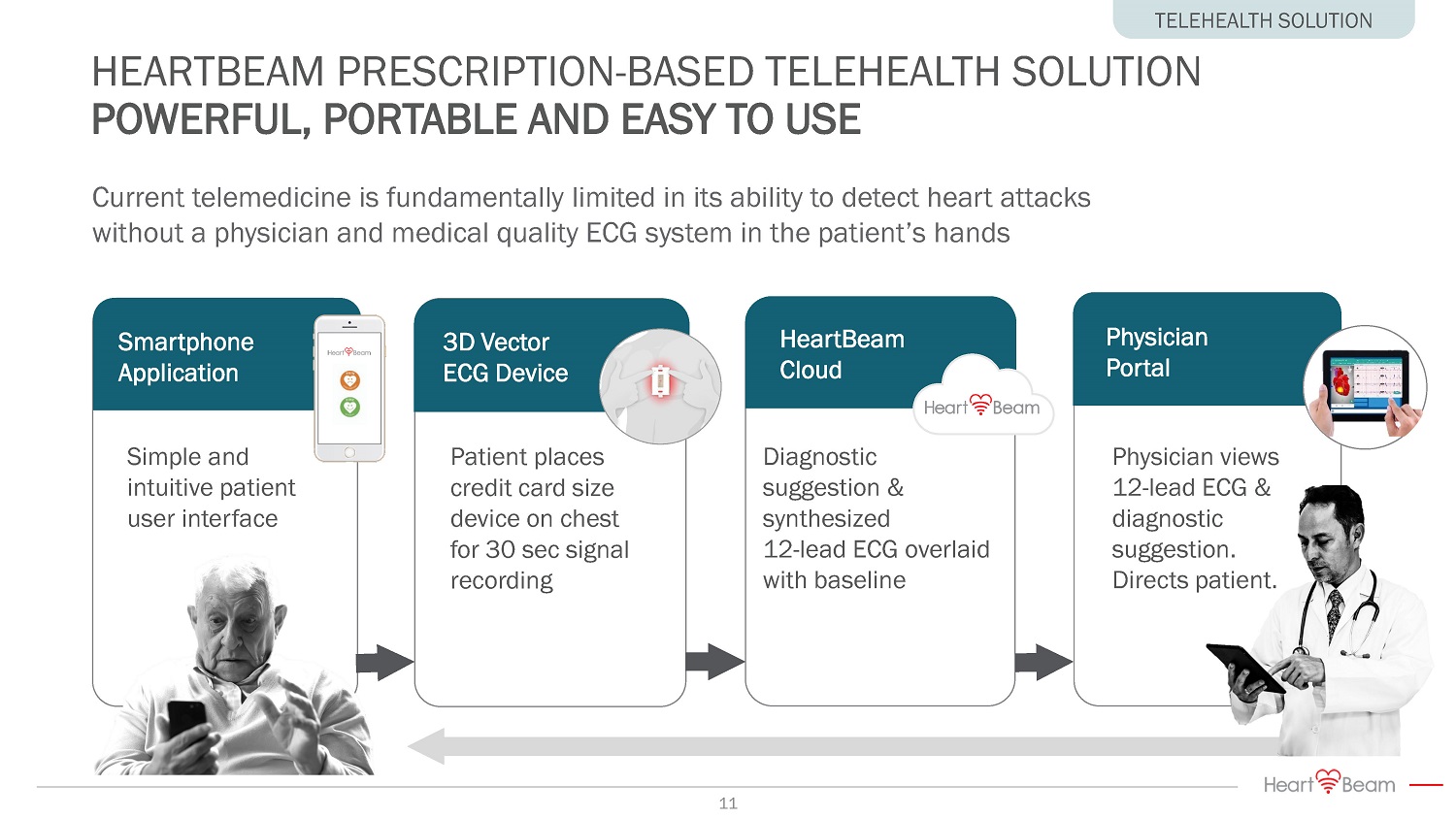
Physician views 12 - lead ECG & diagnostic suggestion. Directs patient. Physician Portal Diagnostic suggestion & synthesized 12 - lead ECG overlaid with baseline HeartBeam Cloud 3D Vector ECG Device Patient places credit card size device on chest for 30 sec signal recording Smartphone Application Simple and intuitive patient user interface Current telemedicine is fundamentally limited in its ability to detect heart attacks without a physician and medical quality ECG system in the patient’s hands HEARTBEAM PRESCRIPTION - BASED TELEHEALTH SOLUTION POWERFUL, PORTABLE AND EASY TO USE TELEHEALTH SOLUTION 11
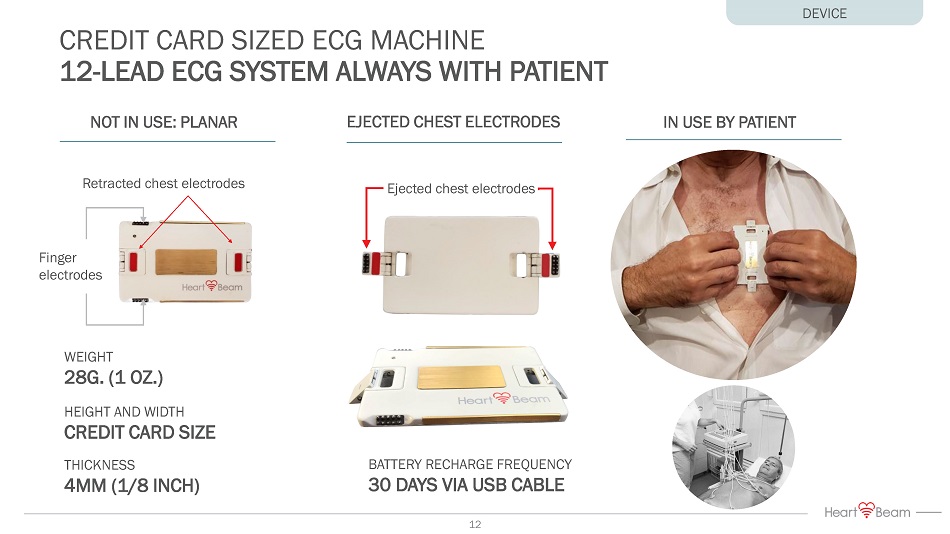
CREDIT CARD SIZED ECG MACHINE 12 - LEAD ECG SYSTEM ALWAYS WITH PATIENT EJECTED CHEST ELECTRODES NOT IN USE: PLANAR IN USE BY PATIENT BATTERY RECHARGE FREQUENCY 30 DAYS VIA USB CABLE WEIGHT 28G. (1 OZ.) HEIGHT AND WIDTH CREDIT CARD SIZE THICKNESS 4MM (1/8 INCH) DEVICE Ejected chest electrodes Retracted chest electrodes Finger electrodes 12 vs.
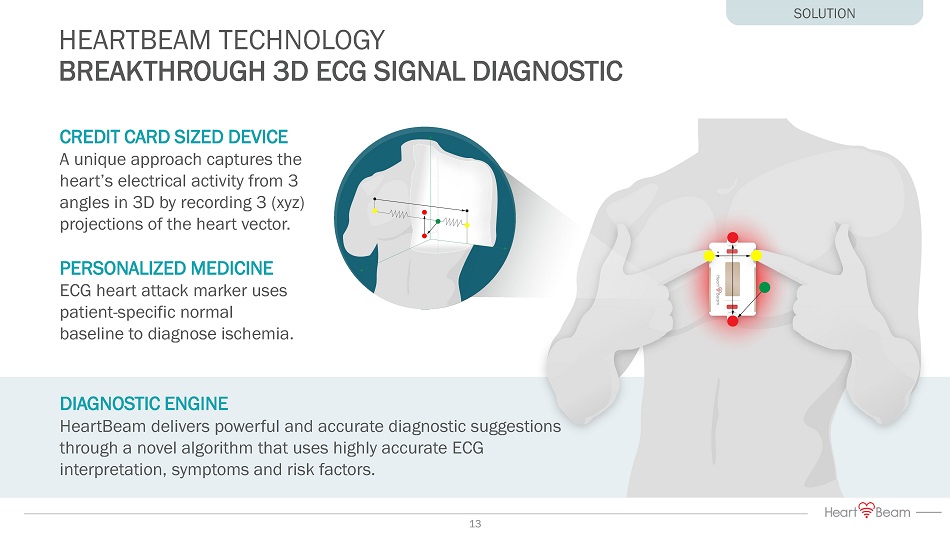
DIAGNOSTIC ENGINE HeartBeam delivers powerful and accurate diagnostic suggestions through a novel algorithm that uses highly accurate ECG interpretation, symptoms and risk factors. HEARTBEAM TECHNOLOGY BREAKTHROUGH 3D ECG SIGNAL DIAGNOSTIC CREDIT CARD SIZED DEVICE A unique approach captures the heart’s electrical activity from 3 angles in 3D by recording 3 (xyz) projections of the heart vector. PERSONALIZED MEDICINE ECG heart attack marker uses patient - specific normal baseline to diagnose ischemia. SOLUTION 13
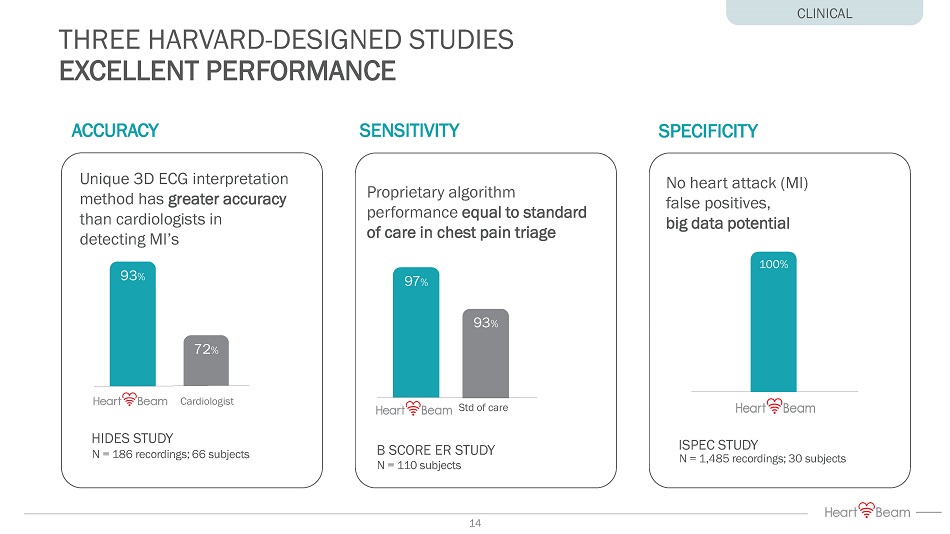
THREE HARVARD - DESIGNED STUDIES EXCELLENT PERFORMANCE HIDES STUDY N = 186 recordings; 66 subjects ACCURACY Unique 3D ECG interpretation method has greater accuracy than cardiologists in detecting MI’s 93 % 72 % Cardiologist B SCORE ER STUDY N = 110 subjects SENSITIVITY Proprietary algorithm performance equal to standard of care in chest pain triage 97 % 93 % Std of care CLINICAL No heart attack (MI) false positives, big data potential ISPEC STUDY N = 1,485 recordings; 30 subjects 100% SPECIFICITY 14

KEY MEDICAL CONTRIBUTORS MIKE GIBSON, MD PETER ZIMETBAUM, MD ALEXEI SHVILKIN, MD NIRAJ VARMA, MD PHILIP SAGER, MD RAJ DASH, MD TOM DEERING, MD JOHN JEFFERIES, MD CHARLIE BROWN, MD JEFFREY J. OYLER, MD BARRY MANGEL, MD TEAM 15
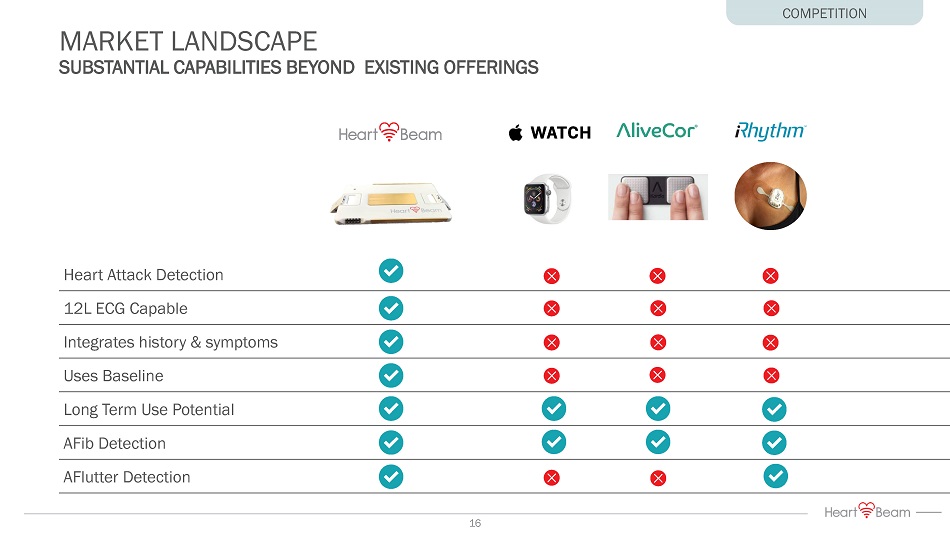
Heart Attack Detection 12L ECG Capable Integrates history & symptoms Uses Baseline Long Term Use Potential AFib Detection AFlutter Detection MARKET LANDSCAPE SUBSTANTIAL CAPABILITIES BEYOND EXISTING OFFERINGS COMPETITION 16
Credit card sized collection device Smartphone app Synthesized baseline + symptomatic 12L ECGs for physician’s review Symptoms report Patient risk factors/history TELEHEALTH PRODUCT DEVELOPMENT AND REGULATORY PLAN FDA 510(K) Predicate devices identified Simple validation study Will provide data on advanced technology features to be introduced in Gen 2 GEN 1 PRODUCT ROLLOUT Telehealth GEN 1 17
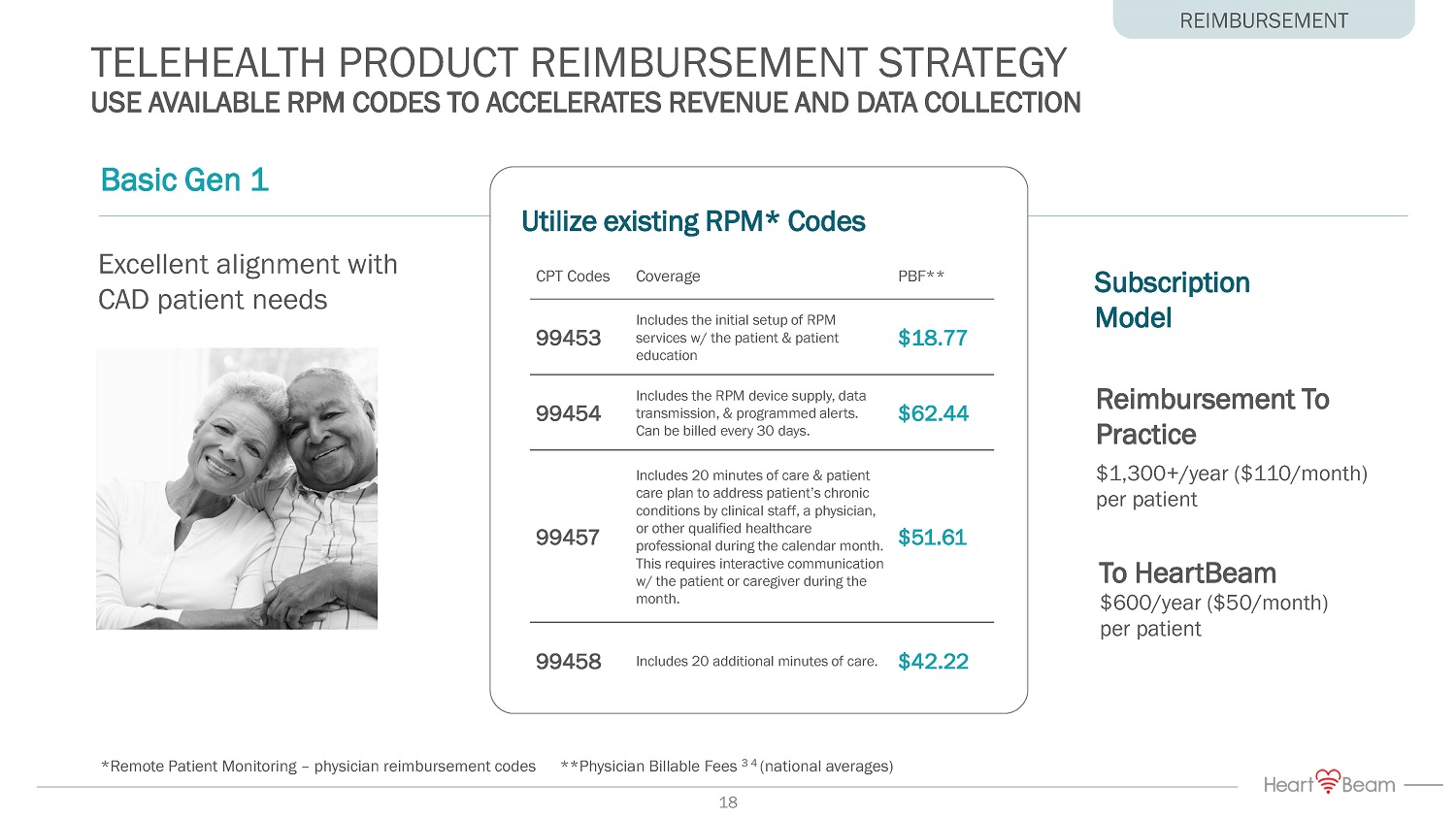
Excellent alignment with CAD patient needs TELEHEALTH PRODUCT REIMBURSEMENT STRATEGY USE AVAILABLE RPM CODES TO ACCELERATES REVENUE AND DATA COLLECTION REIMBURSEMENT Subscription Model To HeartBeam Reimbursement To Practice $600/year ($50/month) per patient $1,300+/year ($110/month) per patient *Remote Patient Monitoring – physician reimbursement codes **Physician Billable Fees 3 4 (national averages) Basic Gen 1 Utilize existing RPM* Codes CPT Codes Coverage PBF** 99453 Includes the initial setup of RPM services w/ the patient & patient education $18.77 99454 Includes the RPM device supply, data transmission, & programmed alerts. Can be billed every 30 days. $62.44 99457 Includes 20 minutes of care & patient care plan to address patient’s chronic conditions by clinical staff, a physician, or other qualified healthcare professional during the calendar month. This requires interactive communication w/ the patient or caregiver during the month. $51.61 99458 Includes 20 additional minutes of care. $42.22 18
HEARTBEAM SOLUTION ER SOFTWARE PRODUCT ER SOLUTION ER Solution for more accurate MI detection ER Software Q3 2022 19
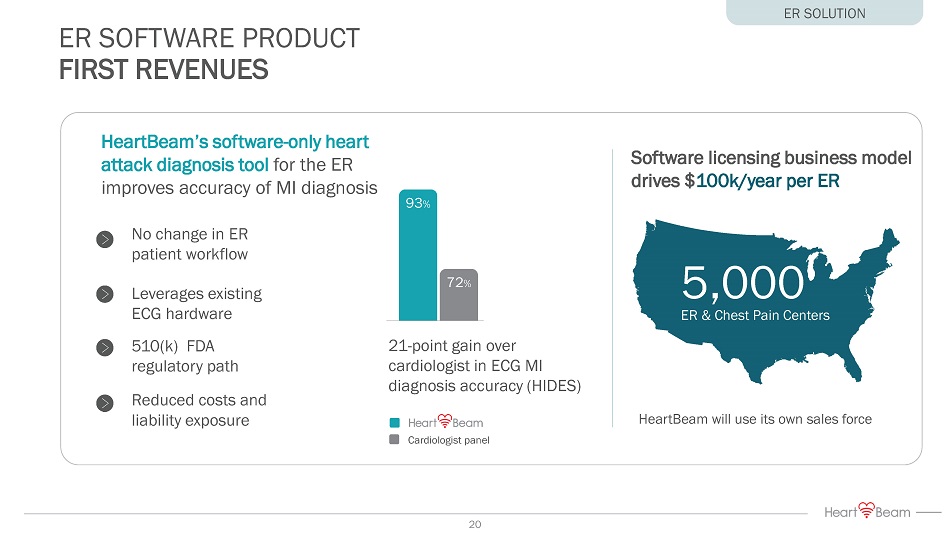
ER SOFTWARE PRODUCT FIRST REVENUES 5,000 ER & Chest Pain Centers Software licensing business model drives $ 100k/year per ER HeartBeam will use its own sales force HeartBeam ’s s oftware - only heart attack diagnosis tool for the ER improves accuracy of MI diagnosis 93 % 72 % Cardiologist panel 21 - point gain over cardiologist in ECG MI diagnosis accuracy (HIDES) No change in ER patient workflow Leverages existing ECG hardware 510(k) FDA regulatory path Reduced costs and liability exposure ER SOLUTION 20
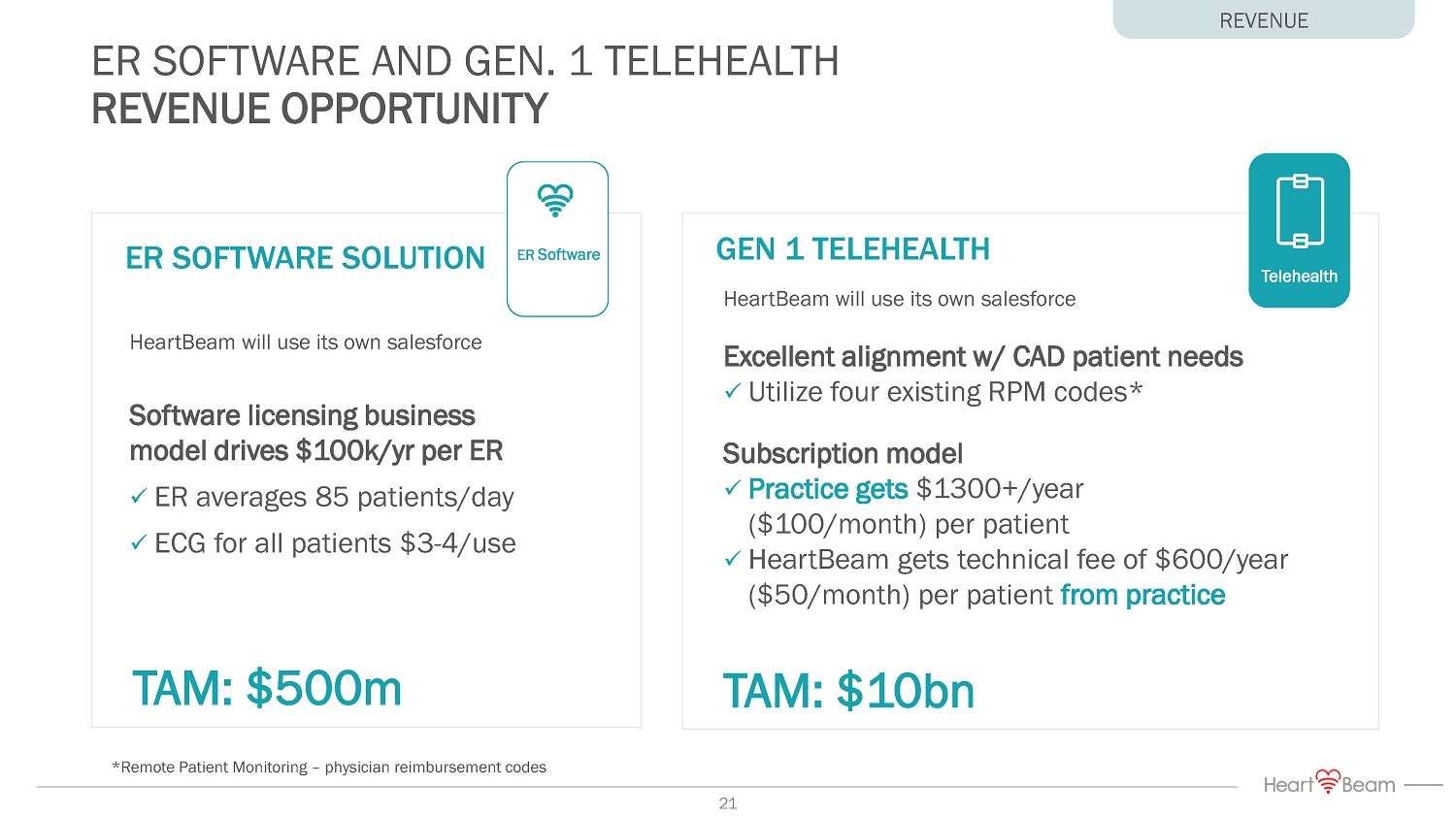
ER SOFTWARE AND GEN. 1 TELEHEALTH REVENUE OPPORTUNITY REVENUE HeartBeam will use its own salesforce Software licensing business model drives $100k/ yr per ER x ER averages 85 patients/day x ECG for all patients $3 - 4/use HeartBeam will use its own salesforce Excellent alignment w/ CAD patient needs x Utilize four existing RPM codes* Subscription model x Practice gets $1300+/year ($100/month) per patient x HeartBeam gets technical fee of $600/year ($50/month) per patient from practice TAM: $10bn *Remote Patient Monitoring – physician reimbursement codes ER SOFTWARE SOLUTION GEN 1 TELEHEALTH ER Software Telehealth TAM: $500m 21
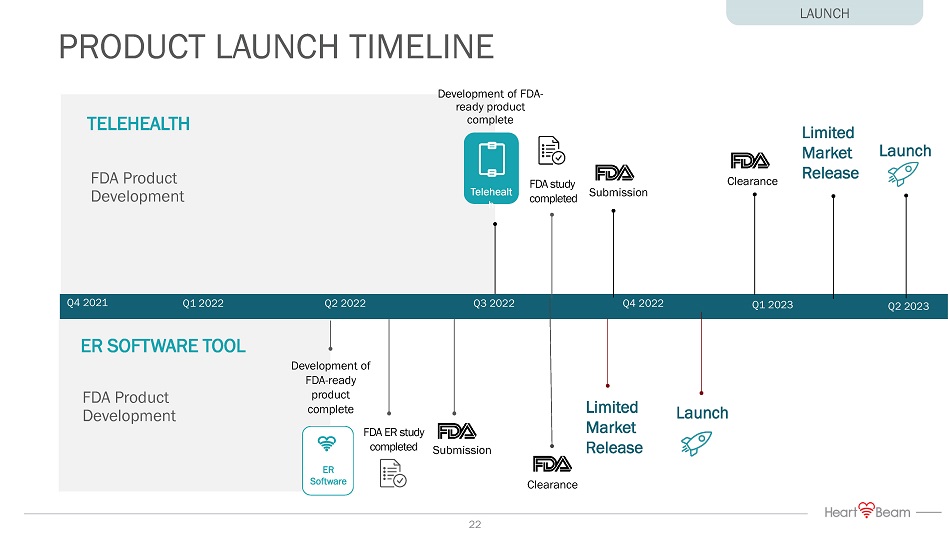
Development of FDA - ready product complete Submission FDA ER study completed Clearance Q1 2022 Q3 2022 Submission FDA study completed Clearance Development of FDA - ready product complete Q4 2022 Q4 2021 ER SOFTWARE TOOL TELEHEALTH Q2 2022 Launch PRODUCT LAUNCH TIMELINE FDA Product Development FDA Product Development Q2 2023 Limited Market Release Launch Q1 2023 Limited Market Release LAUNCH Telehealt h ER Software 22
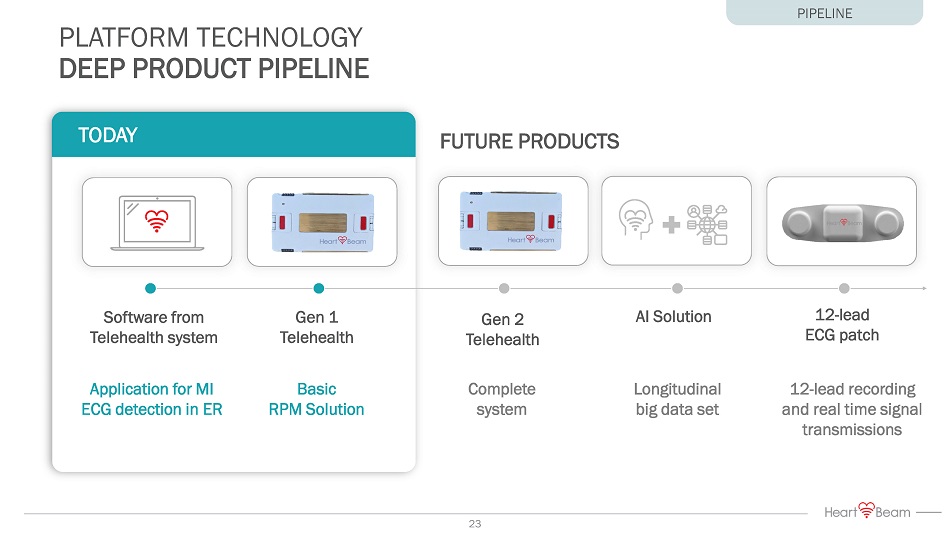
Application for MI ECG detection in ER Basic RPM Solution Gen 1 Telehealth Software from Telehealth system 12 - lead ECG patch PLATFORM TECHNOLOGY DEEP PRODUCT PIPELINE 12 - lead recording and real time signal transmissions AI Solution Longitudinal big data set TODAY FUTURE PRODUCTS Complete system Gen 2 Telehealth PIPELINE 23

Branislav Vajdic, PhD CEO & Founder Alexei Shvilkin, MD Chief Medical Officer Dorin Panescu, PhD Chief Technical Officer Wim Elfrink Board Member Rich Ferrari Executive Chairman Rick Brounstein CFO Alan Baumel COO Jon Hunt, PhD Chief Business Officer George de Urioste Board Member TEAM HIGHLY EXPERIENCED MANAGEMENT SIGNIFICANT EXPERIENCE IN SOFTWARE AND MEDICAL DEVICE PRODUCT DEVELOPMENT 24 Marga Ortigas – Wedekind Board Member
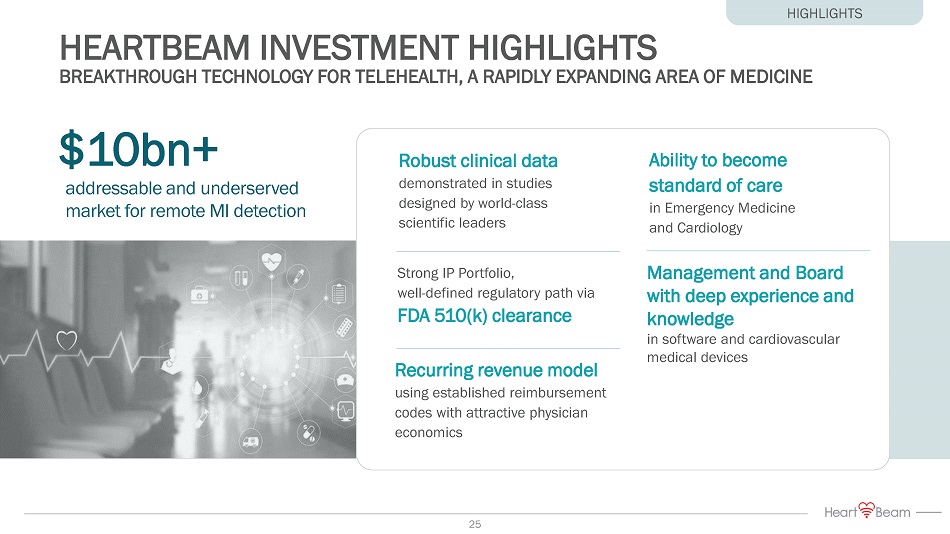
HEARTBEAM INVESTMENT HIGHLIGHTS BREAKTHROUGH TECHNOLOGY FOR TELEHEALTH, A RAPIDLY EXPANDING AREA OF MEDICINE $10bn+ Ability to become standard of care in Emergency Medicine and Cardiology Robust clinical data demonstrated in studies designed by world - class scientific leaders Strong IP Portfolio, well - defined regulatory path via FDA 510(k) clearance Recurring revenue model using established reimbursement codes with attractive physician economics Management and Board with deep experience and knowledge in software and cardiovascular medical devices addressable and underserved market for remote MI detection HIGHLIGHTS 25
Thank you! 26