Exhibit 99.1

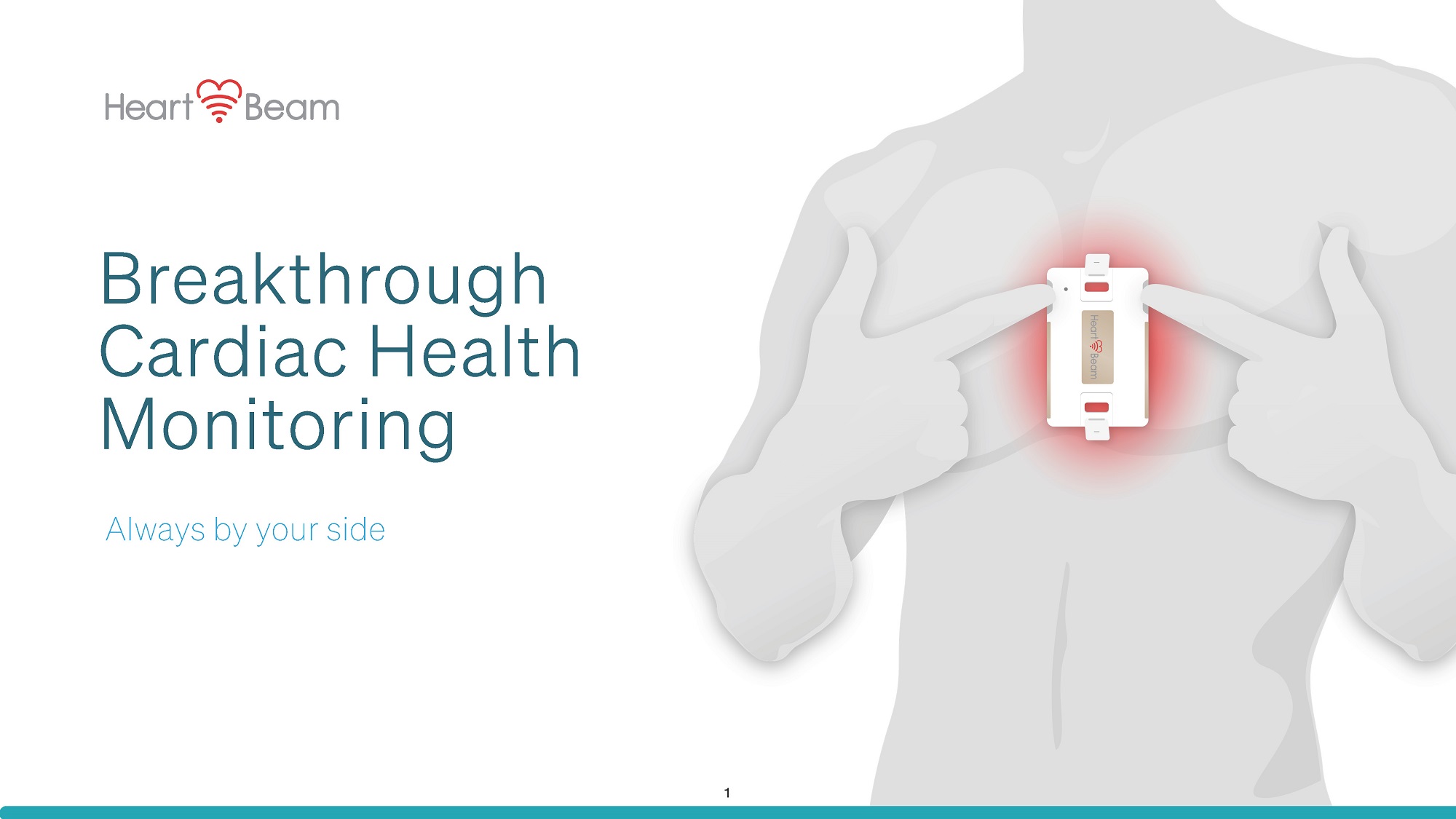
Breakthrough Cardiac Health Monitoring Always by your side 1
Disclaimers This presentation contains forward - looking statements . All statements other than statements of historical fact contained in this presentation, including statements as to the Company’s future results of operations and financial position, planned products and services, business strategy and plans and objectives of management for future operations, are forward - looking statements . These statements involve known and unknown risks, uncertainties, assumptions and other factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements . In some cases, you can identify forward - looking statements by terms such as “may,” “will,” “should,” “expects,” “plans,” anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “aims,” “predicts,” ”potential,” “seeks,” attempts,” “poised“ or “continues” or the negative of these terms or other similar words . These statements are only predictions . The Company has based these forward - looking statements largely on its current expectations and projections about future events and financial trends that it believes may affect its business, financial condition and results of operations . Also, these forward - looking statements represent the Company’s estimates and assumptions only as of the date of this presentation . The Company assumes no obligation to update any forward - looking statements after the date of this presentation . This presentation also contains estimates and other statistical data made by independent parties and by the Company relating to market size and growth and other industry data . This data involves several assumptions and limitations, and you are cautioned not to give undue weight to such estimates . The Company has not independently verified the statistical and other industry data generated by independent parties and contained in this presentation and, accordingly, it cannot guarantee their accuracy or completeness . In addition, projections, assumptions and estimates of its future performance and the future performance of the industries in which it operates are necessarily subject to a high degree of uncertainty and risk due to a variety of factors . These and other factors could cause results to differ materially from those expressed in the estimates made by independent parties and by the Company . For additional risks and uncertainties that could impact the Company's forward - looking statements, please see disclosures contained in HeartBeam’s public filings with the SEC, including the "Risk Factors" in HeartBeam’s Annual Report on Form 10 - K, and which may be viewed at www . sec . gov . The HeartBeam telemedicine technology platform has not yet been evaluated by the FDA and is not approved for clinical use in the USA or other global geographies . 2

Current portable heart monitoring solutions only offer basic monitoring A Practical & Portable Solution T o Replicate 12 - Lead ECG Is Needed Practical, portable devices currently in the market can not replicate the gold standard 12 - lead ECG Single - lead options are limited only to sense heart arrhythmias Only a 12 - lead ECG can suggest heart attack High number of false positives in single - lead devices leads to users ignoring suggestions and overwhelms clinicians Limited applications and predictive value 3

Better diagnosis can improve the management of heart conditions Heart Disease Kills 17.9 Million People Per Year More in - depth and longitudinal data might lead to earlier diagnosis of heart diseases Multiple heart conditions can be diagnosed with richer data set Fast and accurate heart attack suggestion can improve patient outcomes 4
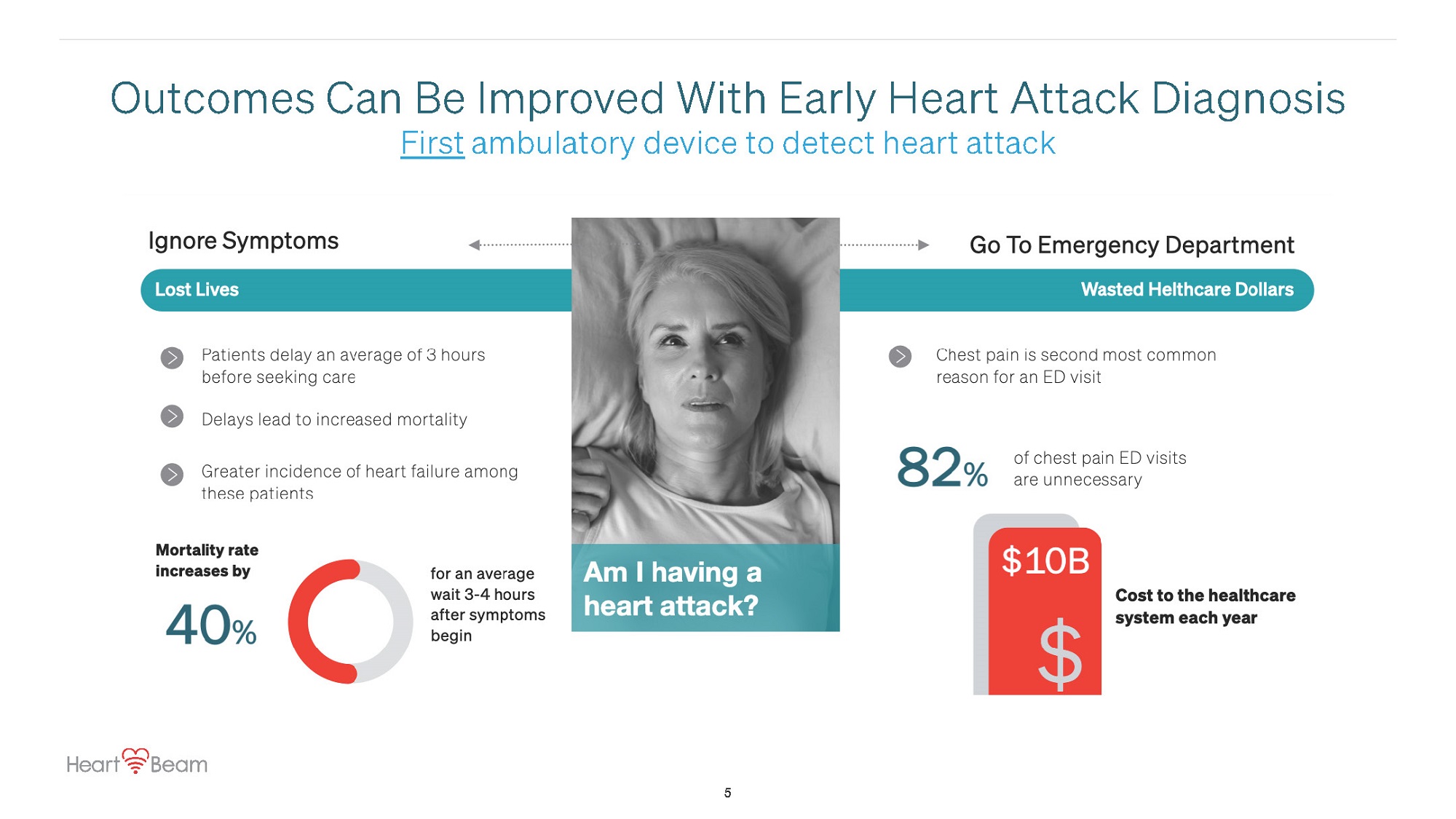
Outcomes Can Be Improved With Early Heart Attack Diagnosis First ambulatory device to detect heart attack 5
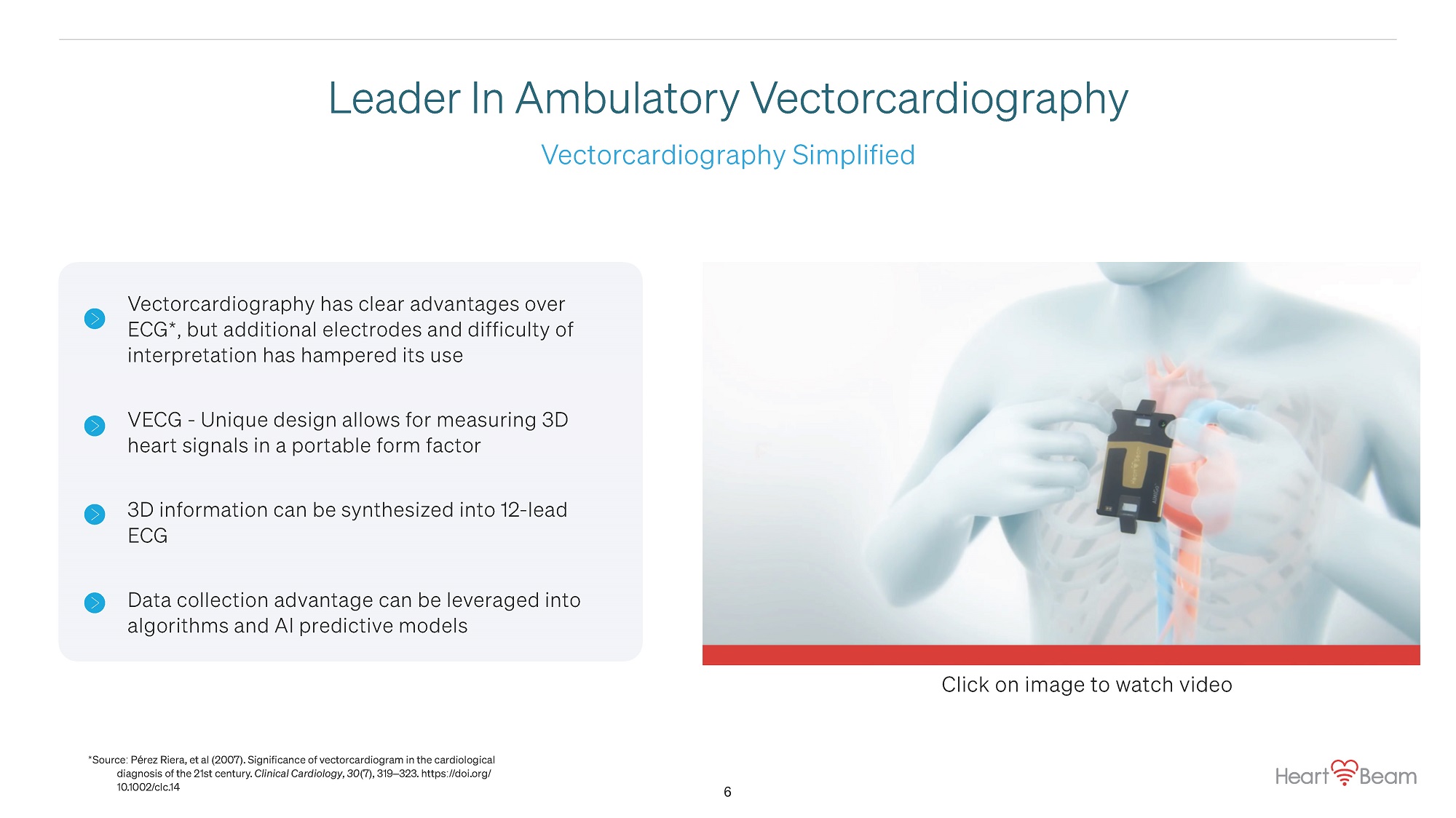
Vectorcardiography Simplified Leader In Ambulatory Vectorcardiography Vectorcardiography has clear advantages over ECG * , but additional electrodes and difficulty of interpretation has hampered its use VECG - Unique design allows for measuring 3D heart signals in a portable form factor 3D information can be synthesized into 12 - lead ECG Data collection advantage can be leveraged into algorithms and AI predictive models Click on image to watch video 6 * Source : P é rez Riera, et al ( 2007 ) . Significance of vectorcardiogram in the cardiological diagnosis of the 21st century. Clinical Cardiology , 30 ( 7 ) , 319 – 323. https :// doi.org / 10.1002 / clc.14
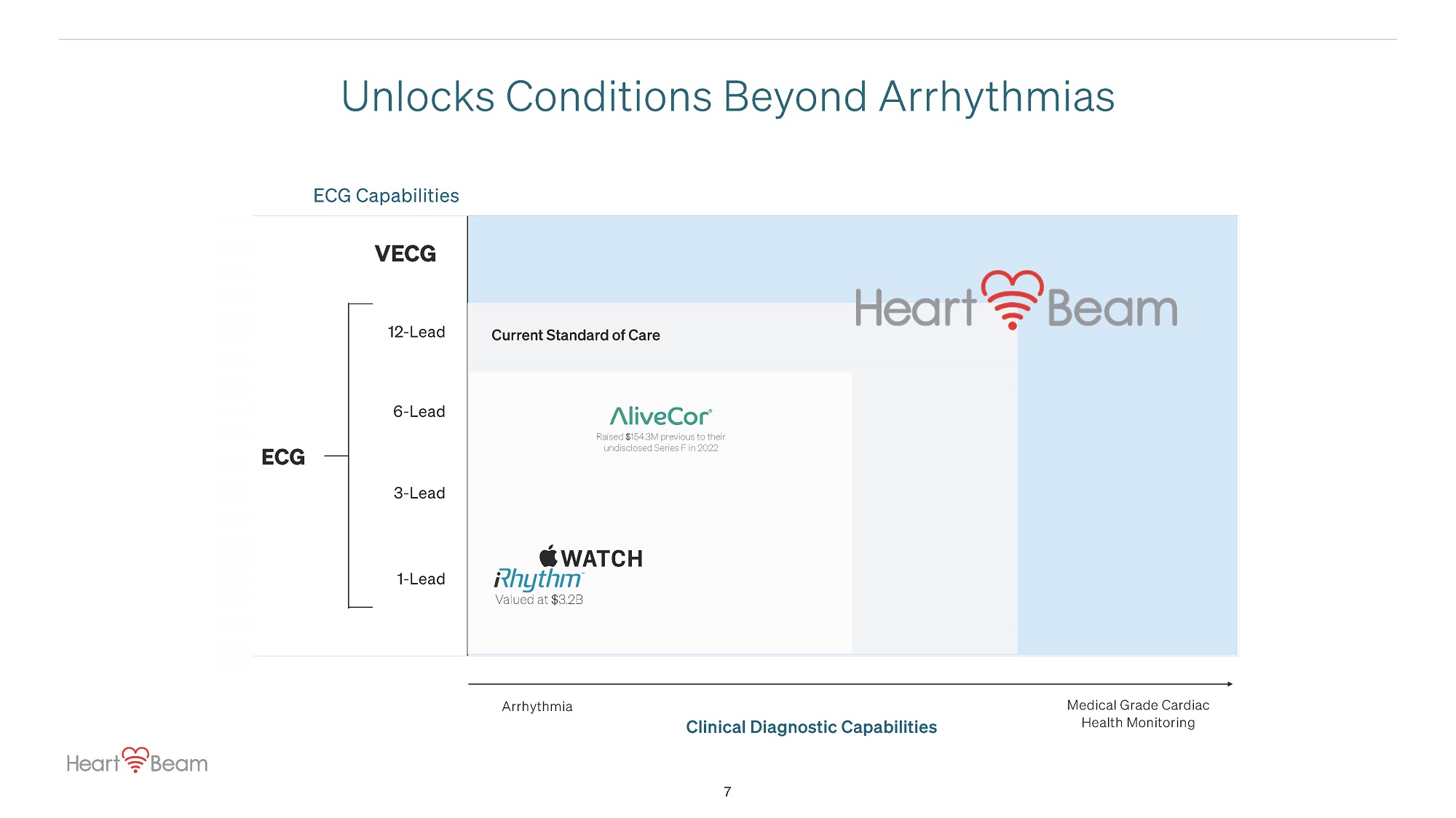
12 - Lead 6 - Lead Clinical Diagnostic Capabilities Medical Grade Cardiac Health Monitoring 3 - Lead ECG Capabilities VECG Unlocks Conditions Beyond Arrhythmias Arrhythmia 1 - Lead Valued at $3.2B Raised $154.3M previous to their undisclosed Series F in 2022 Current Standard of Care ECG 7
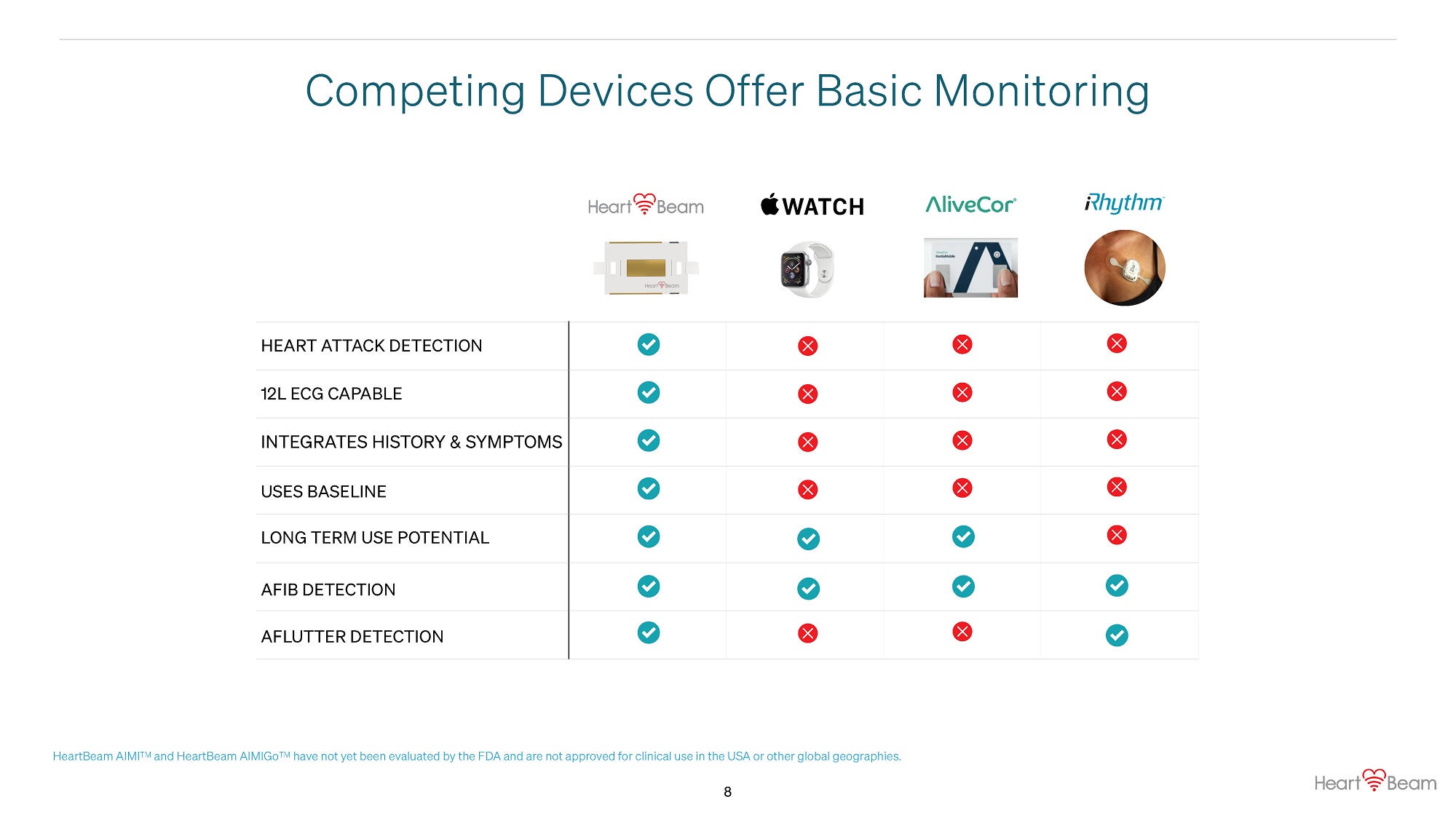
Competing Devices Offer Basic Monitoring HEART ATTACK DETECTION 12L ECG CAPABLE INTEGRATES HISTORY & SYMPTOMS USES BASELINE LONG TERM USE POTENTIAL AFIB DETECTION AFLUTTER DETECTION HeartBeam AIMI TM and HeartBeam AIMIGo TM have not yet been evaluated by the FDA and are not approved for clinical use in the USA or other global geographies. 8
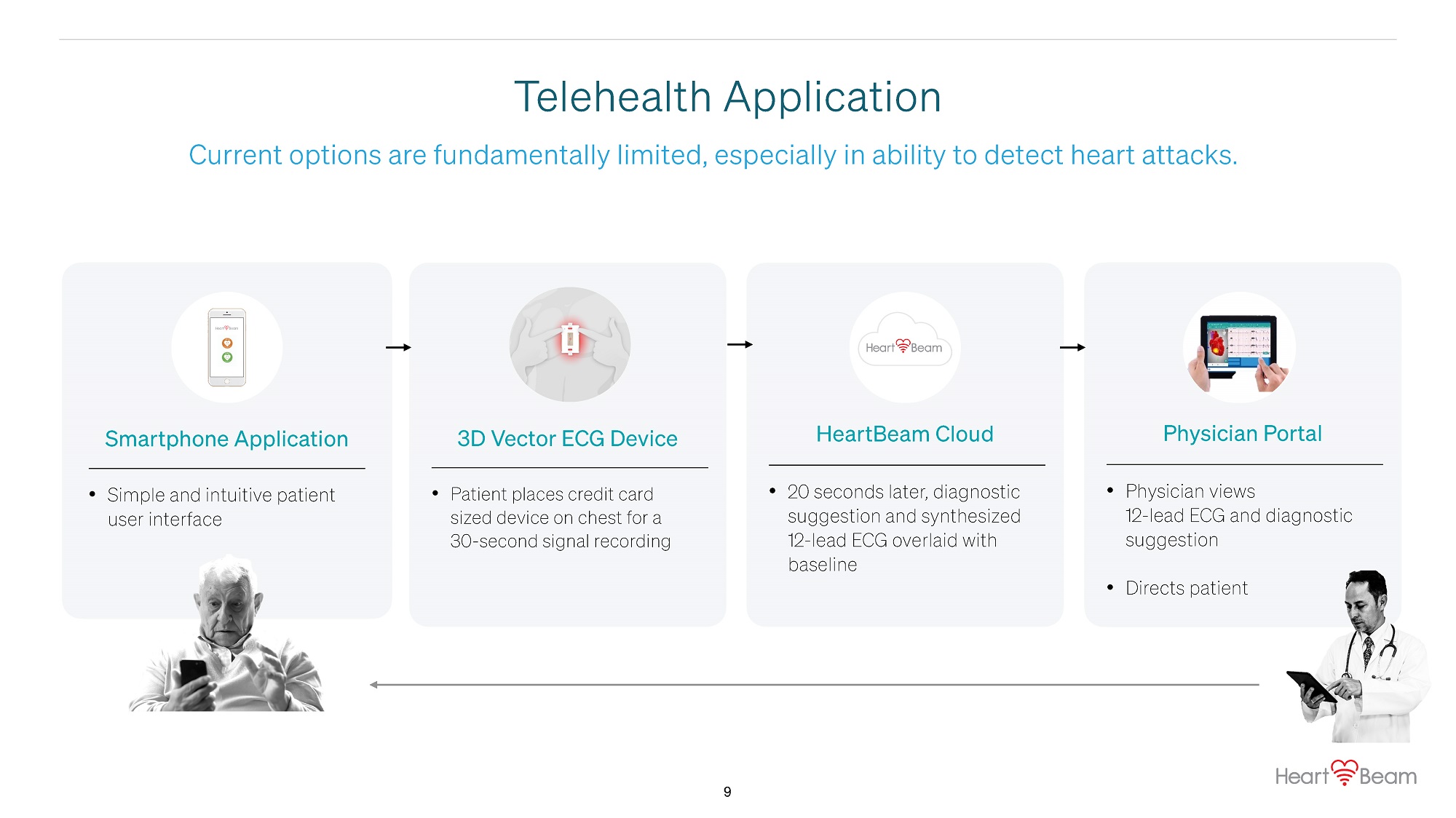
Current options are fundamentally limited, especially in ability to detect heart attacks. Telehealth Application Smartphone Application • Simple and intuitive patient user interface 3D Vector ECG Device • Patient places credit card sized device on chest for a 30 - second signal recording HeartBeam Cloud • 20 seconds later, diagnostic suggestion and synthesized 12 - lead ECG overlaid with baseline Physician Portal • Physician views 12 - lead ECG and diagnostic suggestion • Directs patient 9

WEIGHT 28G. (1 OZ.) HEIGHT AND WIDTH CREDIT CARD SIZE THICKNESS 4MM (1/8 INCH) Flat : Not In Use Ejected Electrodes In Use By Patient Retracted chest electrodes Finger electrodes Ejected chest electrodes RECHARGEABLE BATTERY Practical ECG System 10 12 - Lead ECG System Always With You
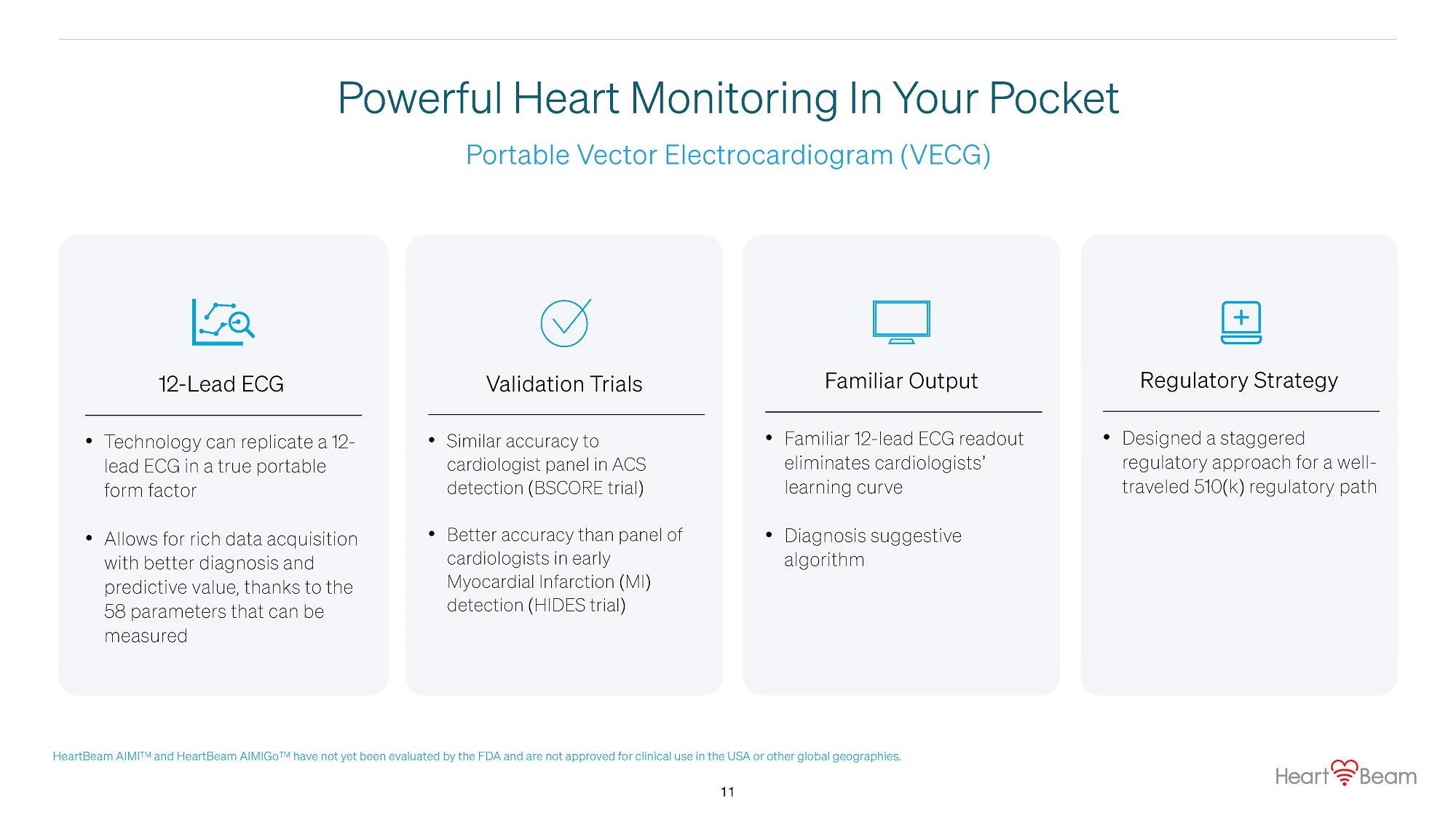
Portable Vector Electrocardiogram ( VECG ) Powerful Heart Monitoring In Your Pocket 12 - Lead ECG • Technology can replicate a 12 - lead ECG in a true portable form factor • Allows for rich data acquisition with better diagnosis and predictive value, thanks to the 58 parameters that can be measured Validation Trials • Similar accuracy to cardiologist panel in ACS detection (BSCORE trial) • Better accuracy than panel of cardiologists in early Myocardial Infarction (MI) detection (HIDES trial) Familiar Output • Familiar 12 - lead ECG readout eliminates cardiologists’ learning curve • Diagnosis suggestive algorithm Regulatory Strategy • 11 Designed a staggered regulatory approach for a well - traveled 510(k) regulatory path HeartBeam AIMI TM and HeartBeam AIMIGo TM have not yet been evaluated by the FDA and are not approved for clinical use in the USA or other global geographies.
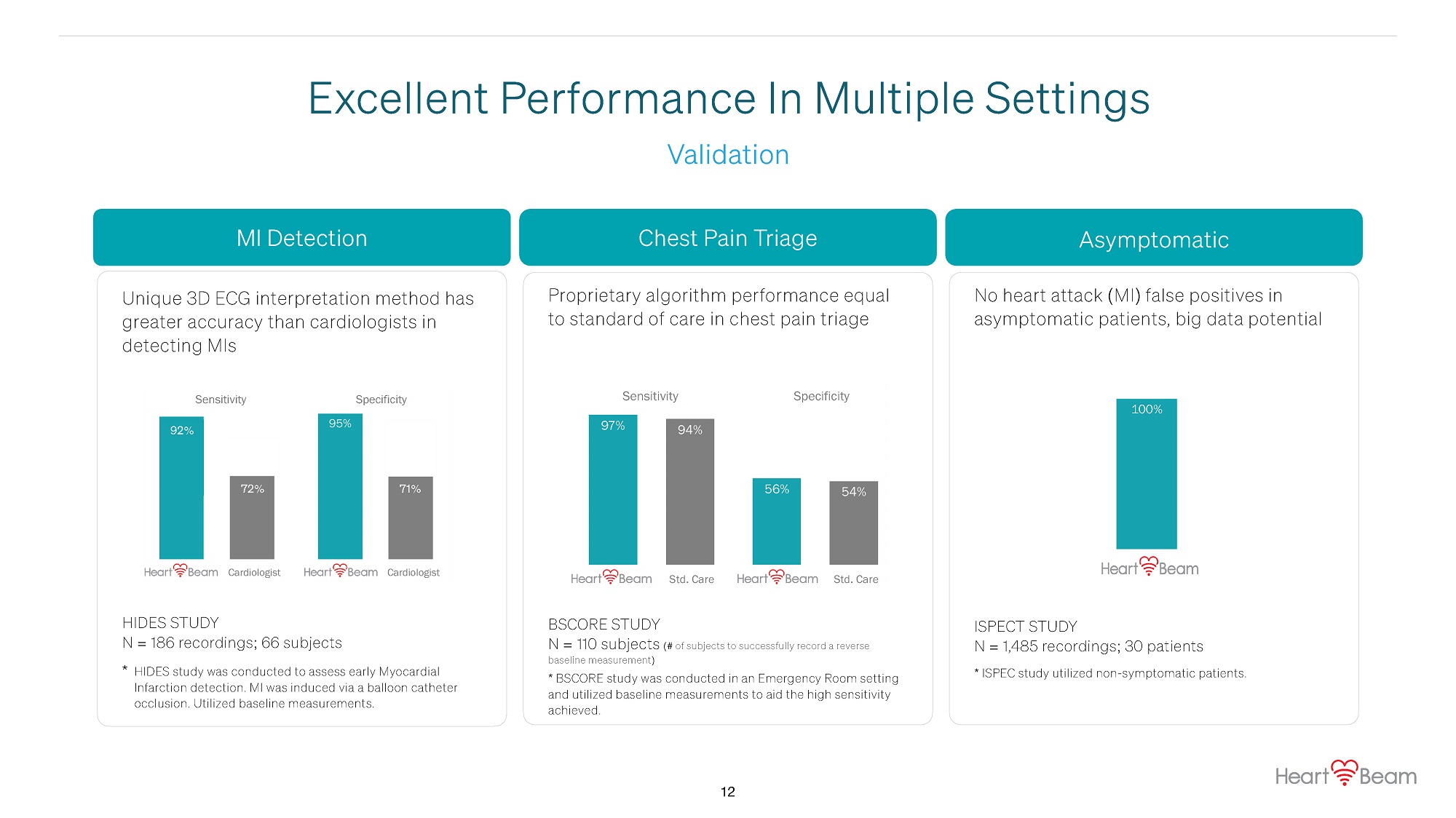
MI Detection Chest Pain Triage Asymptomatic Sensitivity Specificity 93% 95% 83% 70% Cardiologist Cardiologist 97% 56% 94% 54% Sensitivity Specificity Std. Care Std. Care 100% Unique 3D ECG interpretation method has greater accuracy than cardiologists in detecting MIs Proprietary algorithm performance equal to standard of care in chest pain triage No heart attack (MI) false positives in asymptomatic patients, big data potential HIDES STUDY N = 186 recordings; 66 subjects * HIDES study was conducted to assess early Myocardial Infarction detection. MI was induced via a balloon catheter occlusion. Utilized baseline measurements. BSCORE STUDY N = 110 subjects (# of subjects to successfully record a reverse baseline measurement) * BSCORE study was conducted in an Emergency Room setting and utilized baseline measurements to aid the high sensitivity achieved. ISPECT STUDY N = 1,485 recordings; 30 patients * ISPEC study utilized non - symptomatic patients. Validation Excellent Performance In Multiple Settings 93% 95% 70% 83% Cardiologist Cardiologist Sensitivity Specificity 92 % 72 % 71 % 12
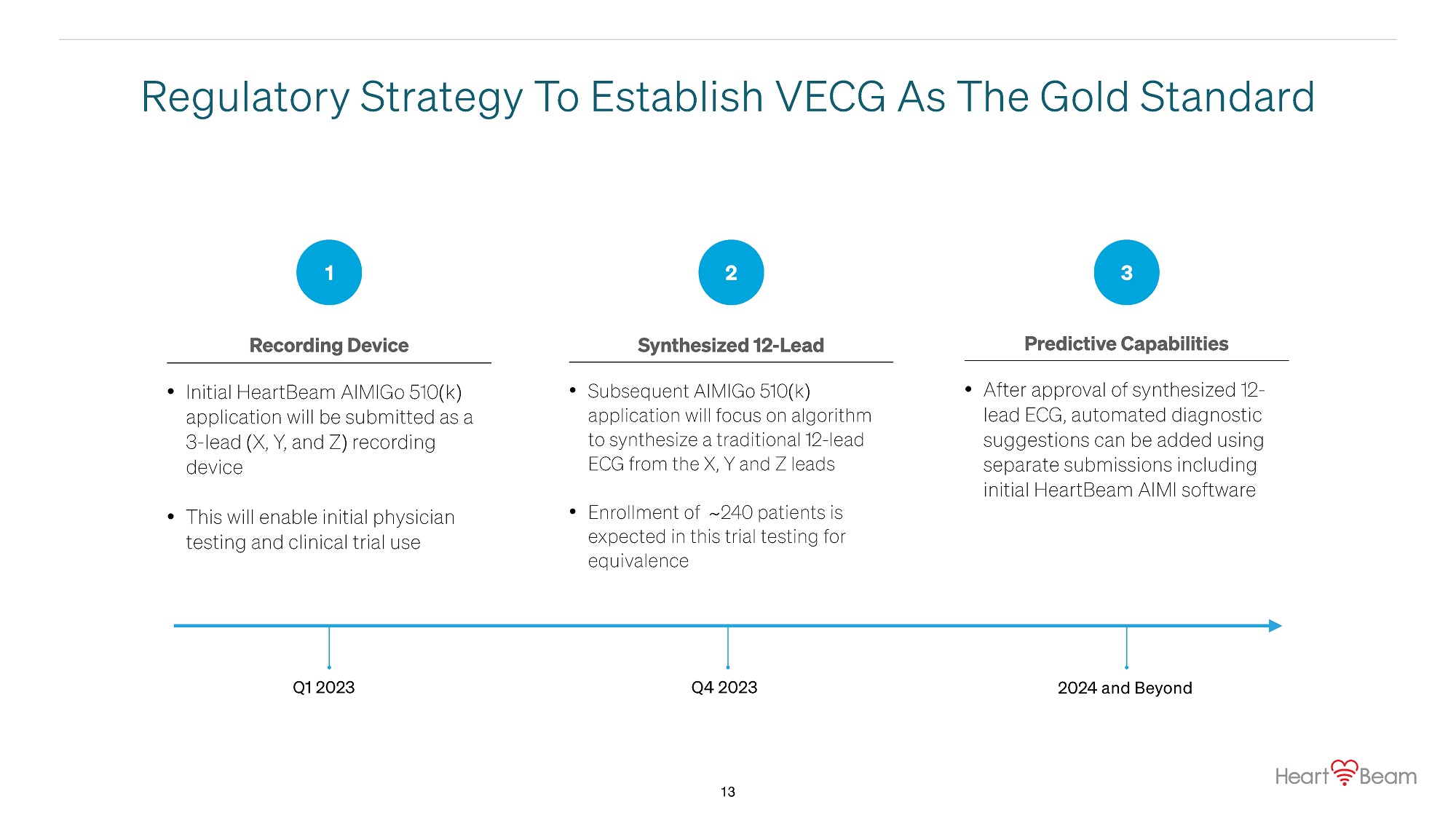
Regulatory Strategy To Establish VECG As The Gold Standard • Initial HeartBeam AIMIGo 510(k) application will be submitted as a 3 - lead (X, Y, and Z) recording device • This will enable initial physician testing and clinical trial use • Subsequent AIMIGo 510(k) application will focus on algorithm to synthesize a traditional 12 - lead ECG from the X, Y and Z leads • Enrollment of ~ 240 patients is expected in this trial testing for equivalence • After approval of synthesized 12 - lead ECG, automated diagnostic suggestions can be added using separate submissions including initial HeartBeam AIMI software 1 2 3 Recording Device Synthesized 12 - Lead Predictive Capabilities Q1 2023 13 Q4 2023 2024 and Beyond
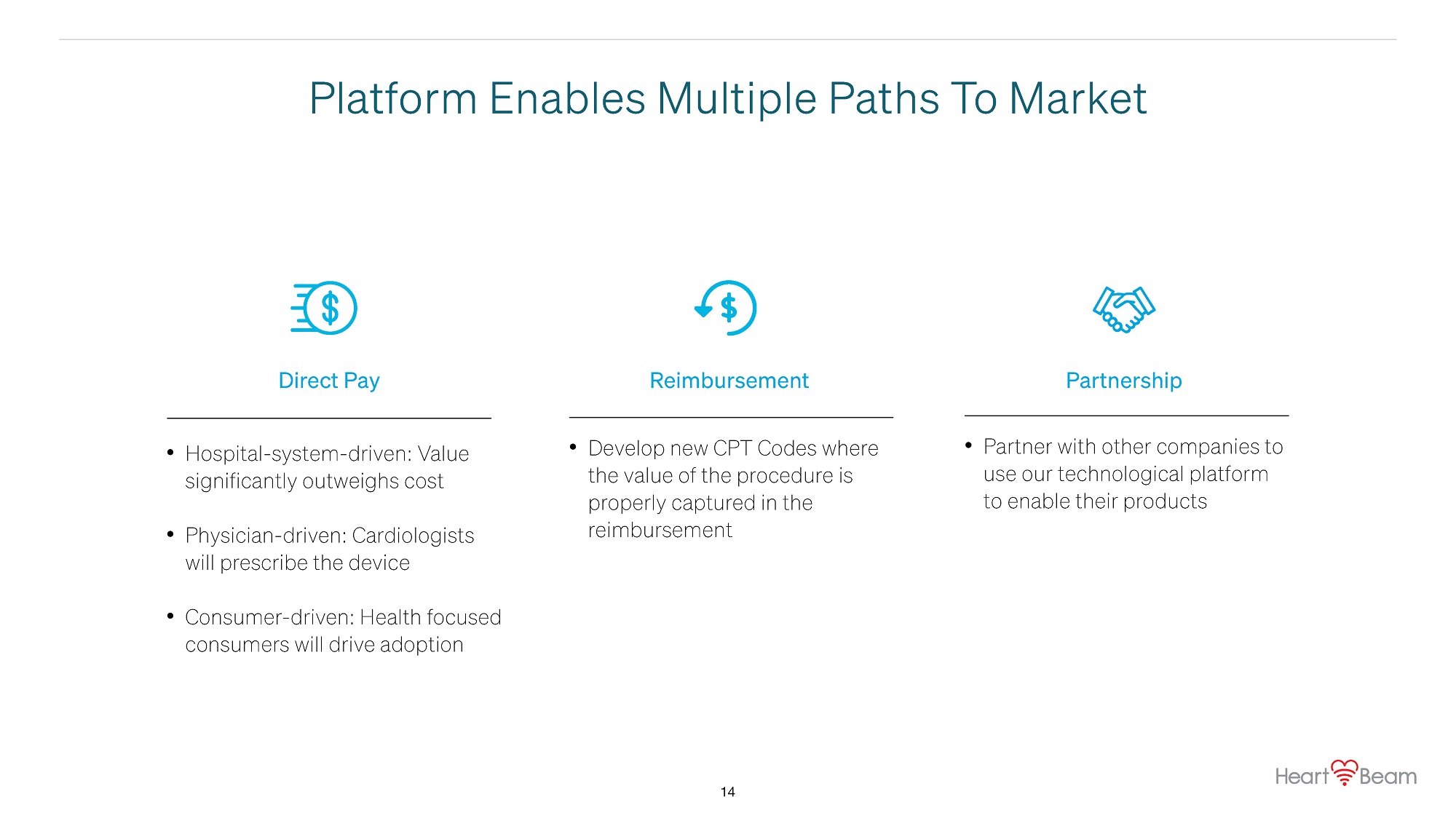
Platform Enables Multiple Paths To Market Direct Pay • Hospital - system - driven: Value significantly outweighs cost • Physician - driven: Cardiologists will prescribe the device • Consumer - driven: Health focused consumers will drive adoption Reimbursement • Develop new CPT Codes where the value of the procedure is properly captured in the reimbursement Partnership • Partner with other companies to use our technological platform to enable their products 14
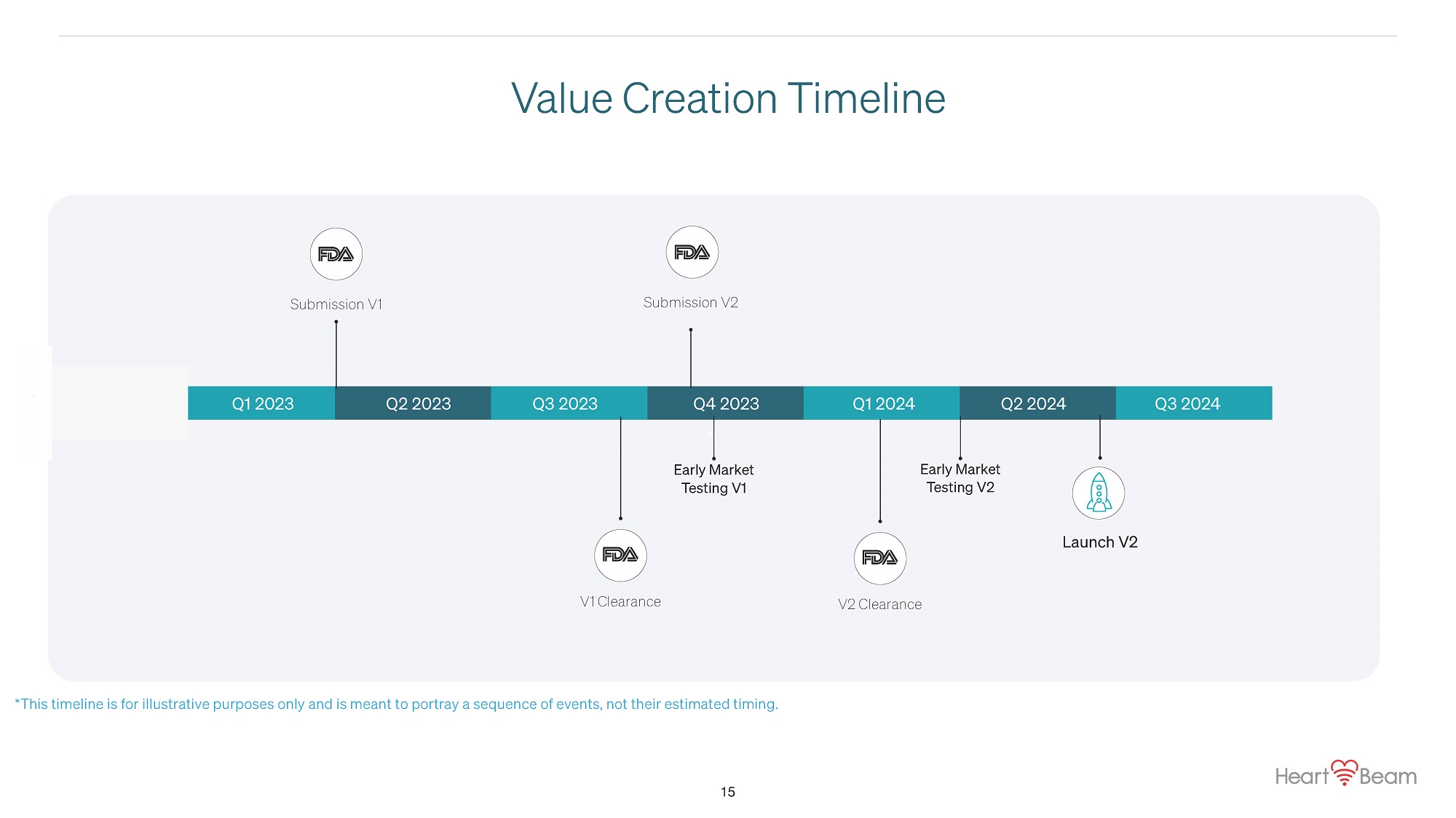
1 Q2 2022 Q1 2023 Q2 2023 Q3 2023 Q4 2023 Q1 2024 Submission V1 Submission V2 Early Market Testing V2 Launch V2 Value Creation Timeline * This timeline is for illustrative purposes only and is meant to portray a sequence of events, not their estimated timing. ` Q2 2024 Q3 2024 V1 Clearance V2 Clearance Early Market Testing V1 15
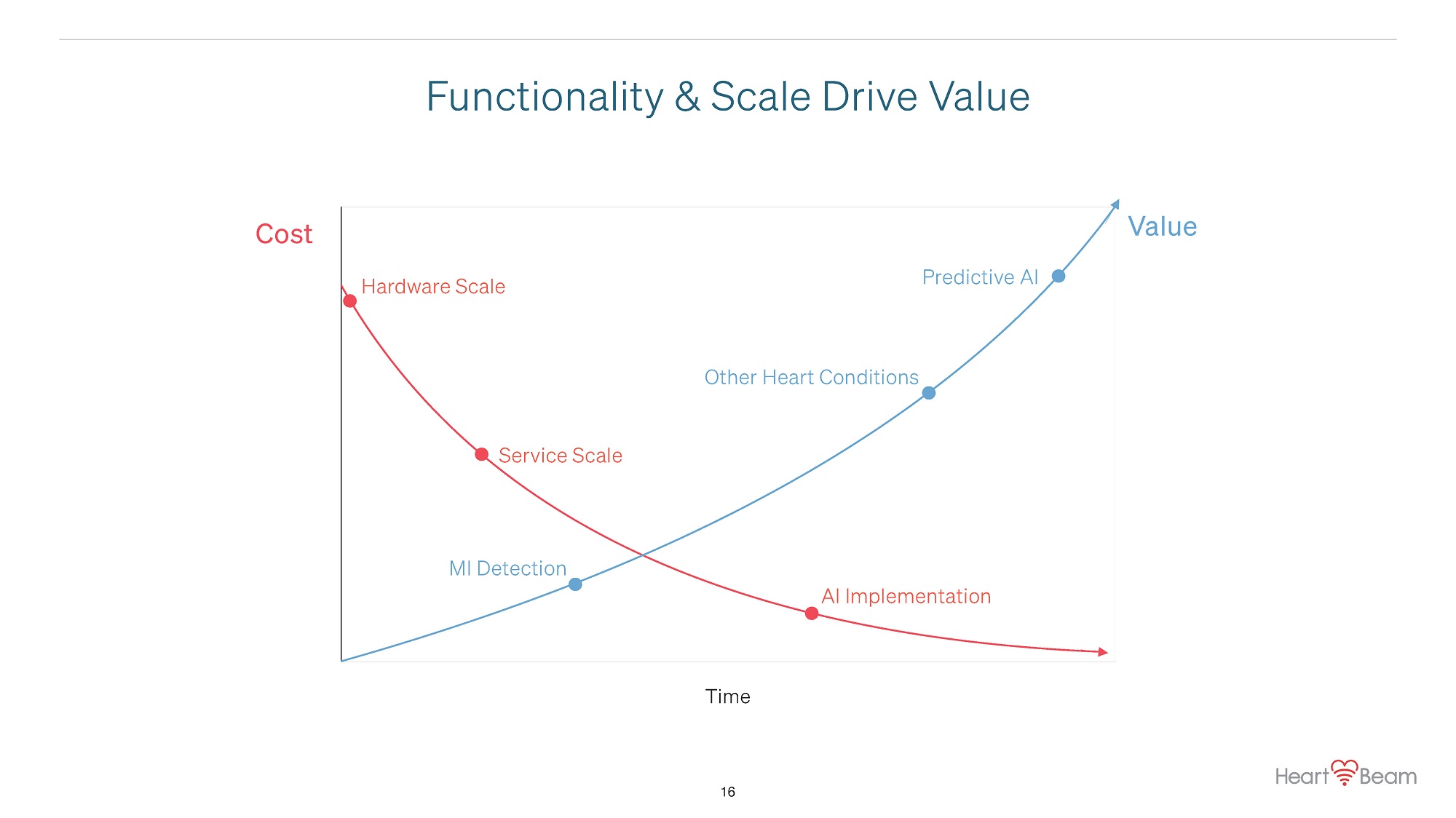
Cost Value Hardware Scale AI Implementation Time Other Heart Conditions Service Scale MI Detection Predictive AI Functionality & Scale Drive Value 16
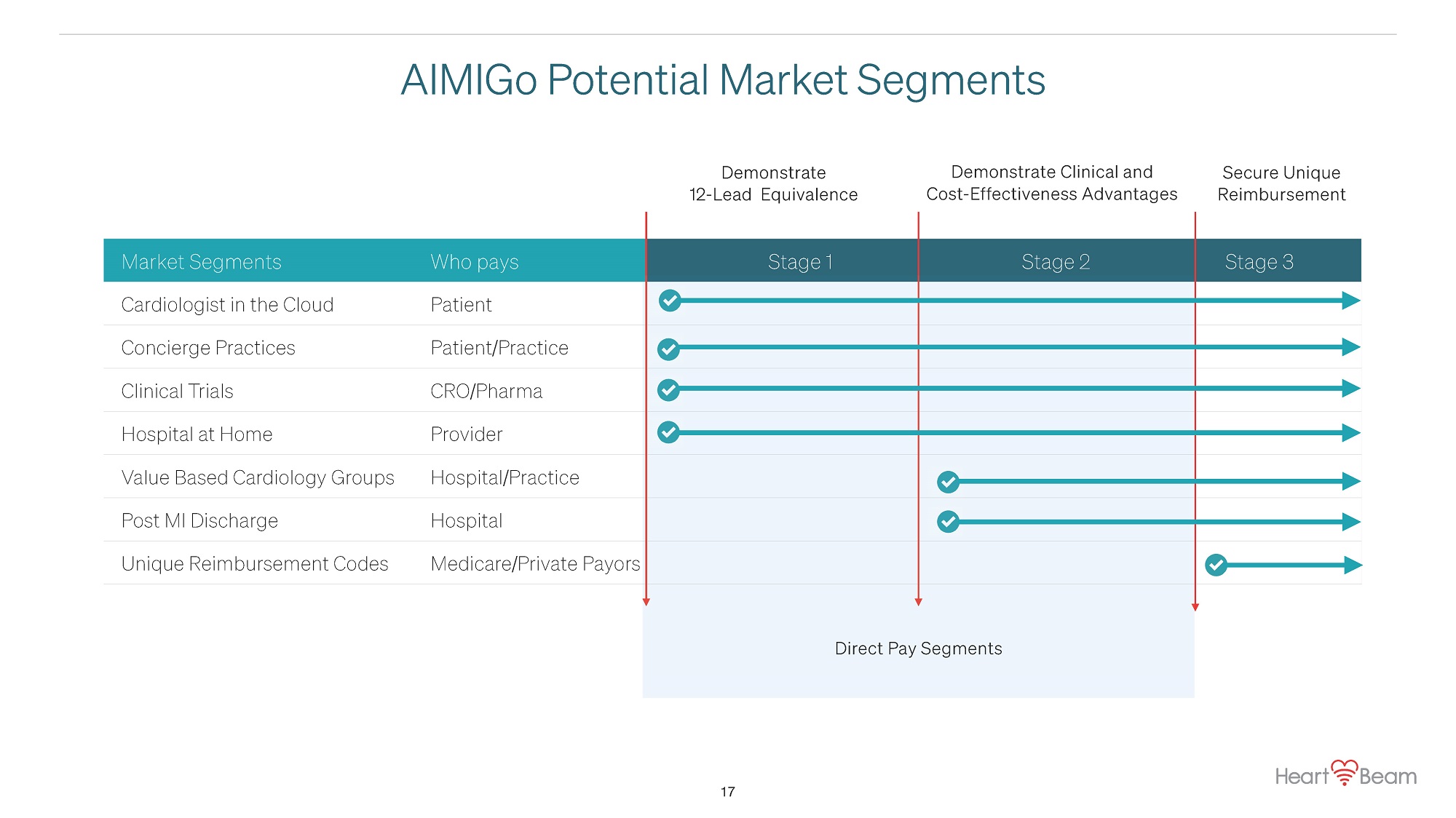
Market Segments Who pays Stage 1 Stage 2 Stage 3 Cardiologist in the Cloud Patient Concierge Practices Patient/Practice Clinical Trials CRO/Pharma Hospital at Home Provider Value Based Cardiology Groups Hospital/Practice Post MI Discharge Hospital Unique Reimbursement Codes Medicare/Private Payors AIMIGo Potential Market Segments Demonstrate 12 - Lead Equivalence Demonstrate Clinical and Cost - Effectiveness Advantages Secure Unique Reimbursement Direct Pay Segments 17

• Multiple granted patents, including foundational resistive network enabling Z - lead • Potential additional patents around improving accuracy and business model • Additional prototypes to lower cost • Foundational patent granted: VECG to 12 - lead upon pressing electrodes with fingers • Capital being allocated to develop prototype • Foundational patent granted : VECG to 12 - lead upon placing band electrodes on chest Intellectual Property & Product Pipeline 18 Leadership Opportunity In Portable VECG Space Card Patch Smart Watch
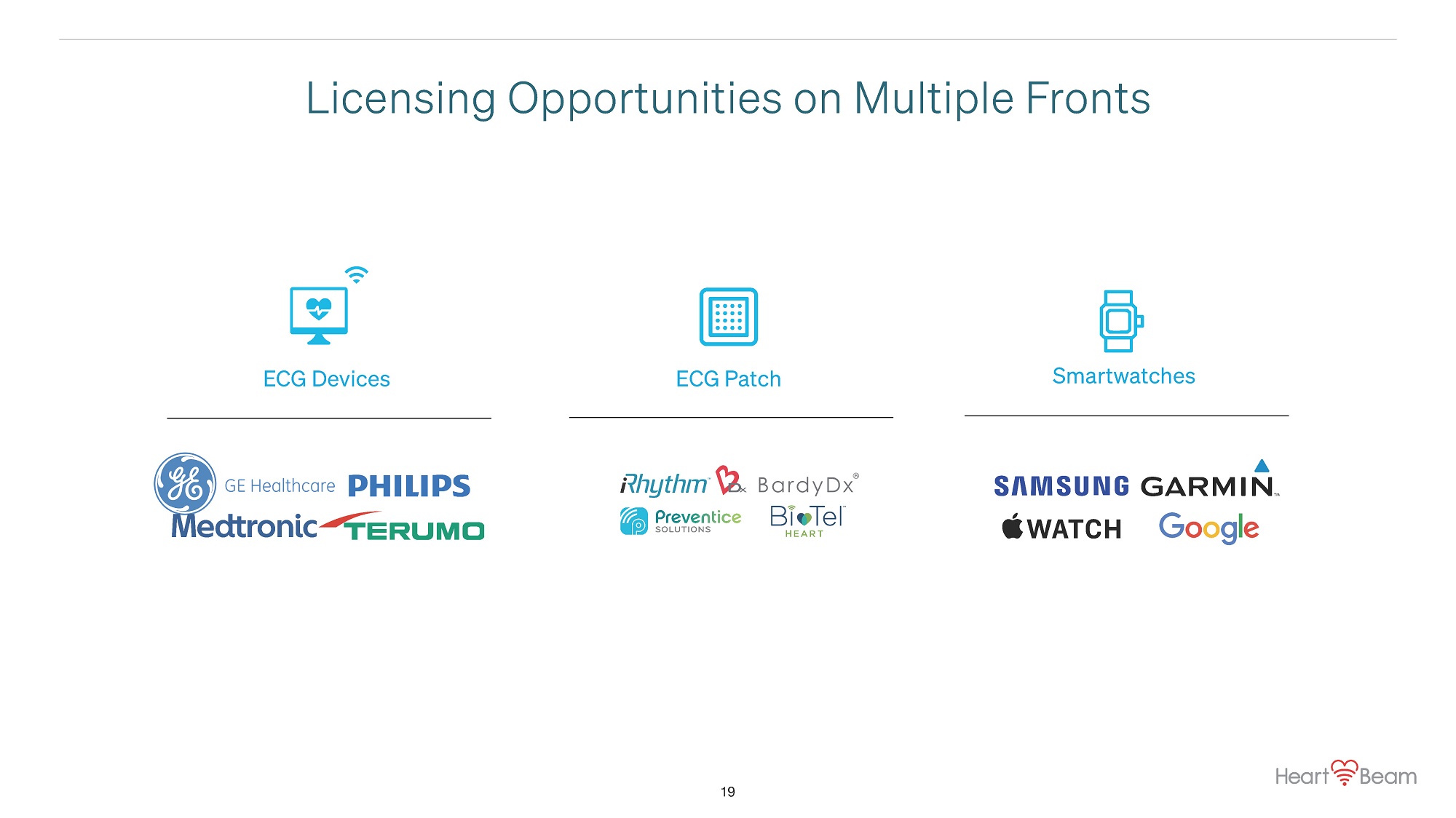
Licensing Opportunities on Multiple Fronts ECG Devices ECG Patch Smartwatches 19

Key Medical Contributors MIKE GIBSON, MD PETER ZIMETBAUM, MD ALEXEI SHVILKIN, MD NIRAJ VARMA, MD STEVE ELLIS, MD PETER FITZGERALD, MD, PhD PHILIP SAGER, MD RAJ DASH, MD JOHN JEFFERIES, MD TOM DEERING, MD CHARLIE BROWN, MD JEFFREY J. OYLER, MD BARRY MANGEL, MD BRETT CANNON, MD World Class Advisors 20

Rich Ferrari Executive Chairman Marga Ortigas – Wedekind Board Member Wim Elfrink Board Member George de Urioste Board Member Branislav Vajdic, PhD CEO & Founder Peter Fitzgerald, MD, PhD Chief Medical Officer Ken Persen Chief Technical Officer Rick Brounstein Chief Financial Officer Significant experience in software & medical device product development Highly Experienced Management Rob Eno President 21
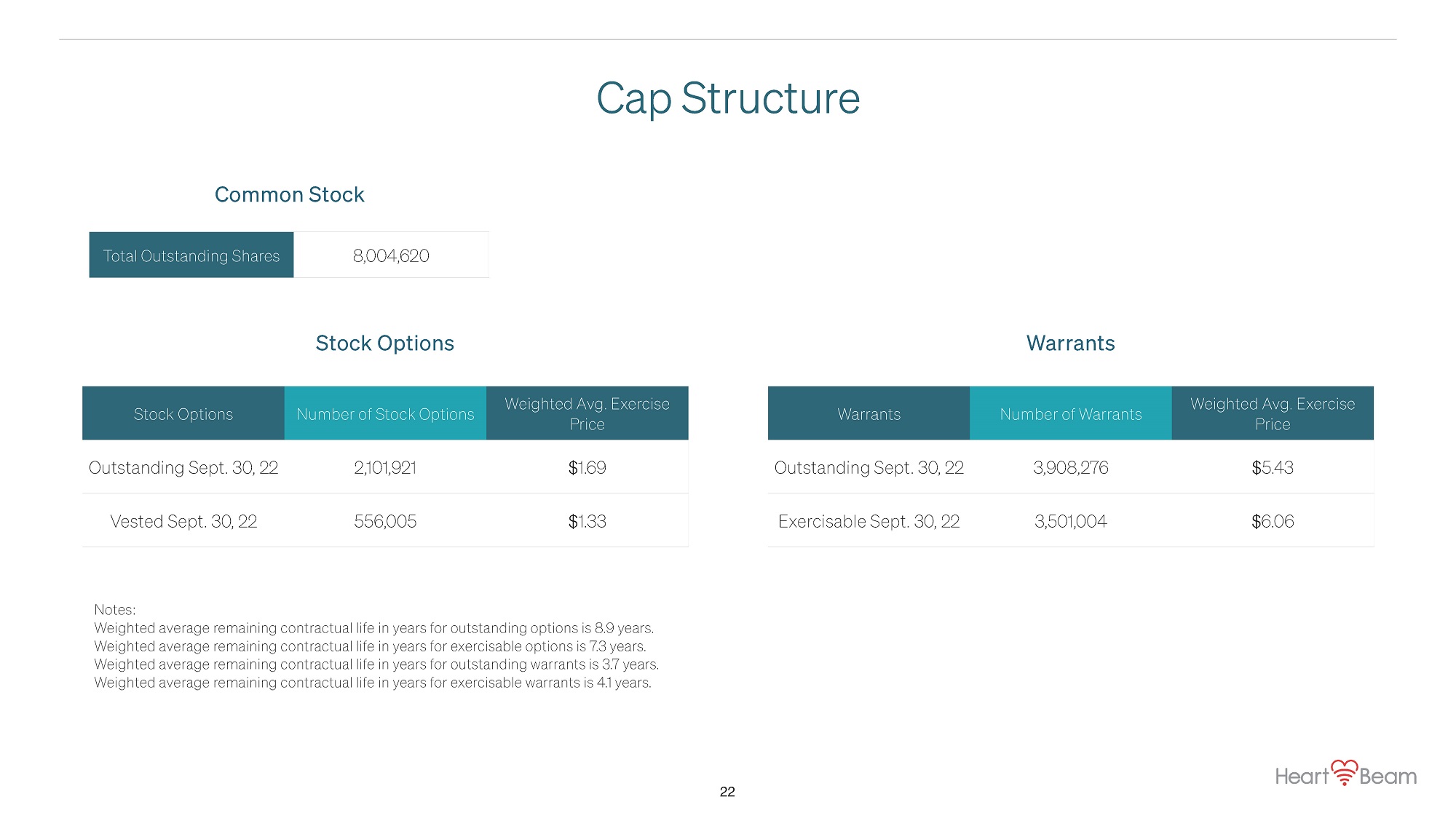
Cap Structure Stock Options Number of Stock Options Weighted Avg. Exercise Price Outstanding Sept. 30, 22 2,101,921 $1.69 Vested Sept. 30, 22 556,005 $1.33 Warrants Number of Warrants Weighted Avg. Exercise Price Outstanding Sept. 30, 22 3,908,276 $5.43 Exercisable Sept. 30, 22 3,501,004 $6.06 Stock Options Warrants Total Outstanding Shares 8,004,620 Notes: Weighted average remaining contractual life in years for outstanding options is 8.9 years. Weighted average remaining contractual life in years for exercisable options is 7.3 years. Weighted average remaining contractual life in years for outstanding warrants is 3.7 years. Weighted average remaining contractual life in years for exercisable warrants is 4.1 years. 22 Common Stock

The Offering Offering Amount $ 15 Million Security Straight Common Stock Price Per Share To Be Determined Offer Type Registered Public Offering 23
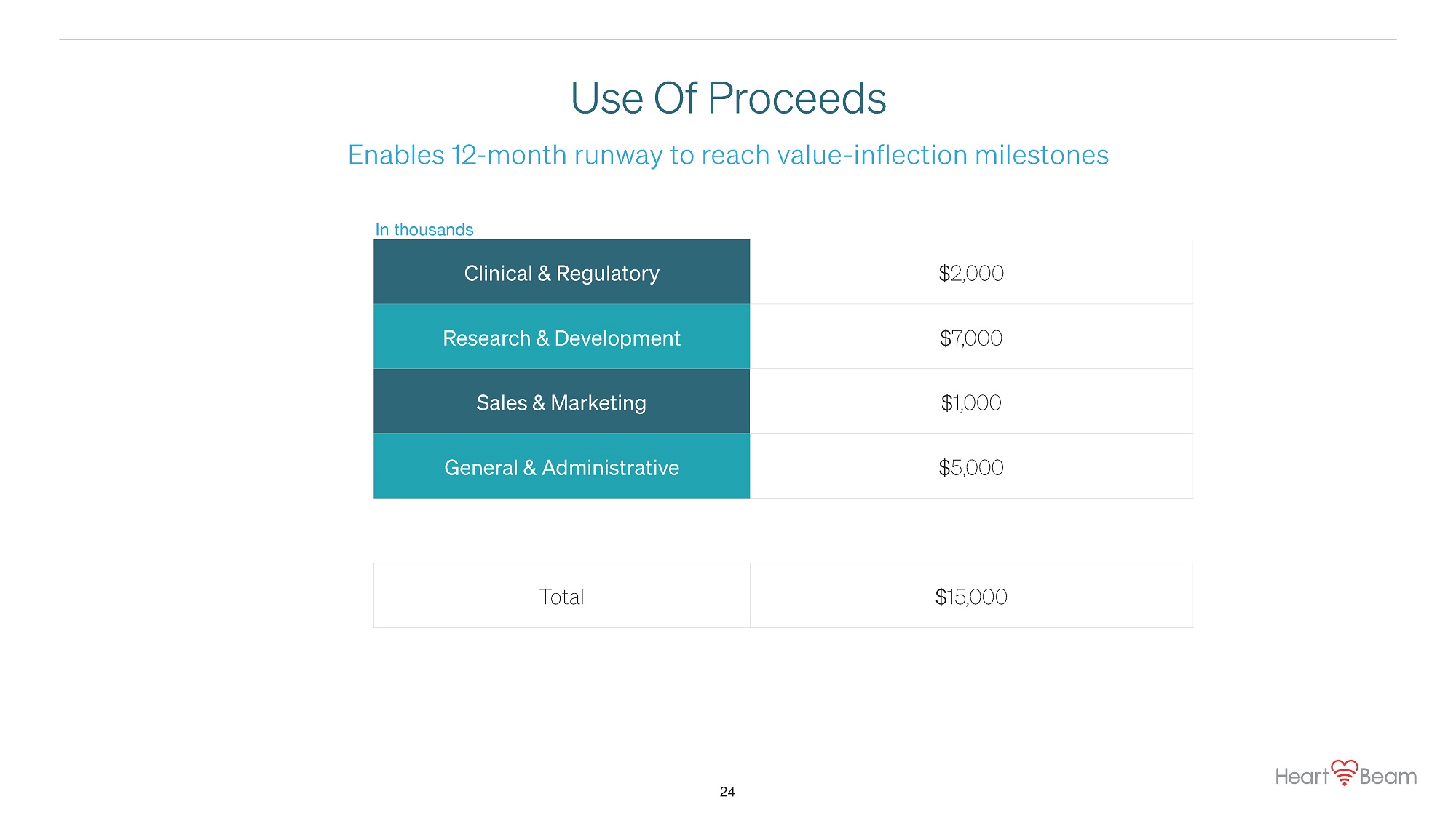
Use Of Proceeds Clinical & Regulatory $2,000 Research & Development $7,000 Sales & Marketing $1,000 General & Administrative $5,000 Total $15,000 In thousands 24 Enables 12 - month runway to reach value - inflection milestones
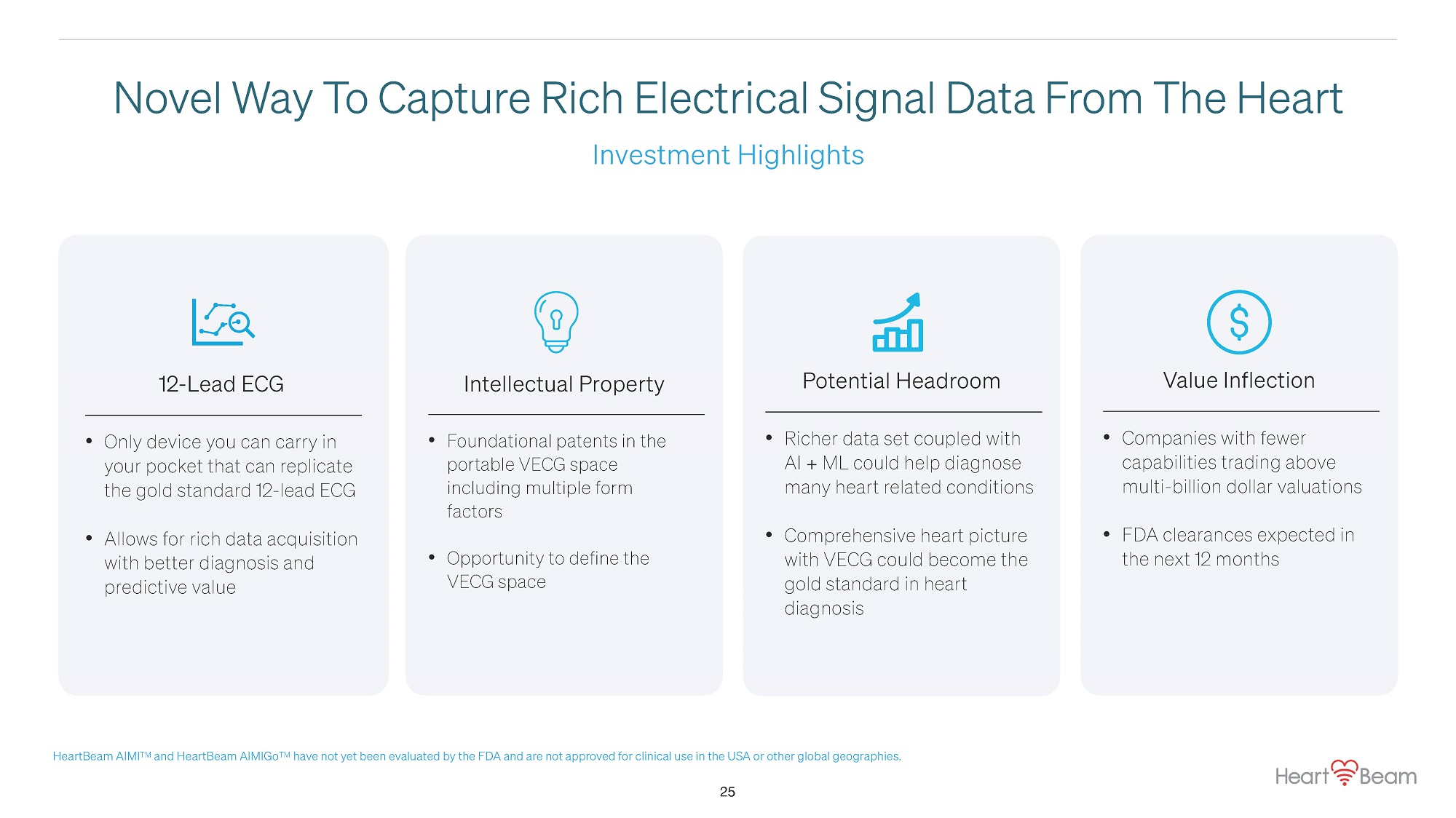
Investment Highlights Novel Way T o Capture Rich Electrical Signal Data From The Heart 12 - Lead ECG • Only device you can carry in your pocket that can replicate the gold standard 12 - lead ECG • Allows for rich data acquisition with better diagnosis and predictive value Intellectual Property • Foundational patents in the portable VECG space including multiple form factors • Opportunity to define the VECG space Potential Headroom • Richer data set coupled with AI + ML could help diagnose many heart related conditions • Comprehensive heart picture with VECG could become the gold standard in heart diagnosis Value Inflection • 25 Companies with fewer capabilities trading above multi - billion dollar valuations • FDA clearances expected in the next 12 months HeartBeam AIMI TM and HeartBeam AIMIGo TM have not yet been evaluated by the FDA and are not approved for clinical use in the USA or other global geographies.
Breakthrough Cardiac Health Monitoring Always by your side 26