|
Prospectus Supplement |
Filed Pursuant to Rule 424(b)(2) |
|||
|
(to Prospectus dated February 10, 2023) |
Registration No. 333-269520 |
HEARTBEAM, INC.
1,000,000 Shares of Common Stock
|
We are offering and selling to an investor 1,000,000 shares of our Common Stock, par value $0.0001, at an offering price of $1.50 pursuant to this prospectus and the accompanying base prospectus. The sales of the shares of Common Stock will be made in accordance with a Securities Purchase Agreement, dated as of May 2, 2023, as amended, by and between us and the investor named therein (the “Securities Purchase Agreement”). Our common stock and warrants are listed on The NASDAQ Capital Market under the symbol “BEAT” and “BEATW”, respectively. On May 3, 2023, the last reported sale price of our common stock on The NASDAQ Capital Market was $2.31 per share. |
|
Per Share(1) |
Total |
Proceeds, |
|||||||
|
Offering price |
$ |
1.50 |
$ |
1,500,000 |
$ |
1,400,000 |
|||
Investing in our securities involves a high degree of risk, including that the trading price of our common stock has been subject to volatility. See “Risk Factors” beginning on page S-11 of this prospectus supplement, page 9 of the accompanying base prospectus and under similar headings in the documents incorporated by reference into this prospectus supplement and the accompanying base prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus supplement is May 4, 2023
|
Page |
||
|
Prospectus Supplement |
||
|
S-ii |
||
|
S-1 |
||
|
S-11 |
||
|
S-12 |
||
|
S-13 |
||
|
S-13 |
||
|
S-13 |
||
|
S-13 |
||
|
S-14 |
||
|
S-14 |
||
|
S-15 |
||
Prospectus
|
ii |
||
|
1 |
||
|
9 |
||
|
10 |
||
|
11 |
||
|
12 |
||
|
16 |
||
|
22 |
||
|
24 |
||
|
25 |
||
|
26 |
||
|
28 |
||
|
28 |
||
|
28 |
||
|
29 |
S-i
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying base prospectus are part of a registration statement that we filed with the U.S. Securities and Exchange Commission, or SEC, utilizing a “shelf” registration process. This document is in two parts. The first part is this prospectus supplement, which describes the specific terms of this offering and also adds to and updates information contained in the accompanying base prospectus and the documents incorporated by reference herein. The second part, the accompanying base prospectus, provides more general information. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying base prospectus or any document incorporated by reference therein filed prior to the date of this prospectus supplement, you should rely on the information in this prospectus supplement; provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date-for example, a document incorporated by reference in the accompanying base prospectus-the statement in the document having the later date modifies or supersedes the earlier statement.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
You should rely only on the information contained in this prospectus supplement or the accompanying base prospectus or incorporated by reference herein. We have not authorized anyone to provide you with information that is different. The information contained in this prospectus supplement or the accompanying base prospectus, or incorporated by reference herein or therein is accurate only as of the respective dates thereof, regardless of the time of delivery of this prospectus supplement and the accompanying base prospectus or of any sale of our Common Stock. It is important for you to read and consider all information contained in this prospectus supplement and the accompanying base prospectus, including the documents incorporated by reference herein and therein, in making your investment decision. You should also read and consider the information in the documents to which we have referred you in the sections entitled “Where You Can Find More Information” and “Incorporation of Certain Information by Reference” in this prospectus supplement and in the accompanying base prospectus, respectively.
We are offering to sell, and seeking offers to buy, the securities offered by this prospectus supplement only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the accompanying base prospectus and the offering of the securities offered by this prospectus supplement in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying base prospectus must inform themselves about, and observe any restrictions relating to, the offering of the Common Stock and the distribution of this prospectus supplement and the accompanying base prospectus outside the United States. This prospectus supplement and the accompanying base prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying base prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
When we refer to “BEAT,” “we,” “our,” “us” and the “Company” in this prospectus, we mean HeartBeam, Inc., unless otherwise specified. When we refer to “you,” we mean the holders of the applicable series of securities.
S-ii
The following information below is only a summary of more detailed information included elsewhere in, or incorporated by reference into, this prospectus supplement and the accompanying base prospectus, and should be read together with the information contained or incorporated by reference in other parts of this prospectus supplement and the accompanying base prospectus. This summary highlights selected information about us and this offering. This summary may not contain all of the information that may be important to you. Before making a decision to invest in our securities, you should read carefully all of the information contained in or incorporated by reference into this prospectus supplement and the accompanying base prospectus, including the information set forth under the caption “Risk Factors” in this prospectus supplement and the accompanying base prospectus as well as the documents incorporated herein by reference, which are described under “Where You Can Find More Information; Incorporation by Reference” in this prospectus supplement. Unless otherwise indicated or unless the context requires otherwise, this prospectus includes the accounts of HeartBeam, Inc., a Delaware corporation and its wholly-owned subsidiaries, collectively referred to as “we”, “us”, “our”, “HeartBeam” or the “Company”.
Overview
Overview
Company Overview
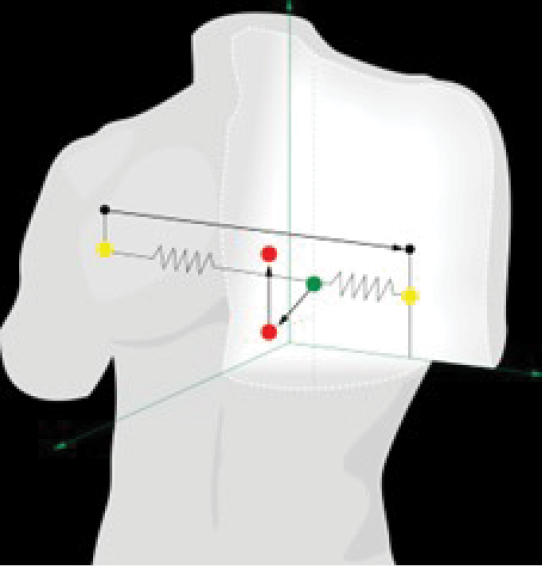
We are a medical technology company primarily focusing on developing and commercializing higher resolution ambulatory Electrocardiogram (“ECG”) solutions that enable the detection and monitoring of cardiac disease both inside and outside a healthcare facility setting. Our ability to develop higher resolution ECG solutions is achieved through the development of our proprietary and patented Vector Electrocardiography (“VECG”) technology platform. Our VECG is capable of developing three-dimensional (“3D”) vector images of cardiac electrical activity by displaying the spatial locations of ECG waveforms that demonstrated in early studies to deliver equal or superior diagnostic capability than traditional hospital-based ECG systems.
Our aim is to deliver innovative, ambulatory cardiac health monitoring technologies that can be used for patients anywhere, especially where critical cardiac care decisions need to be made on a more timely basis. Our products (hereinafter “Product” or “Products”) require Food and Drug Administration (“FDA”) clearance and have not been cleared for marketing.
We believe our Products and services will benefit many stakeholders, including patients, healthcare providers, and healthcare payors. We are developing our telehealth Product (“HeartBeam AIMIGoTM”), to address the rapidly growing telehealth market. HeartBeam AIMIGo is comprised of a credit card sized electrocardiogram device and powerful cloud-based diagnostic expert software systems. We intend to show that our easy to use device (without external electrodes) provides signals equivalent to a standard 12L device, and therefore will have a number of applications for ambulatory use. We believe that we are uniquely positioned to play a central role in cardiac remote monitoring including high-risk coronary artery disease patients, because the initial studies have shown that our ischemia detection system may be more accurate than existing ambulatory monitoring solutions. Coronary artery disease (“CAD”) patients are at increased risk for a heart attack or Myocardial Infarction (“MI”).
S-1
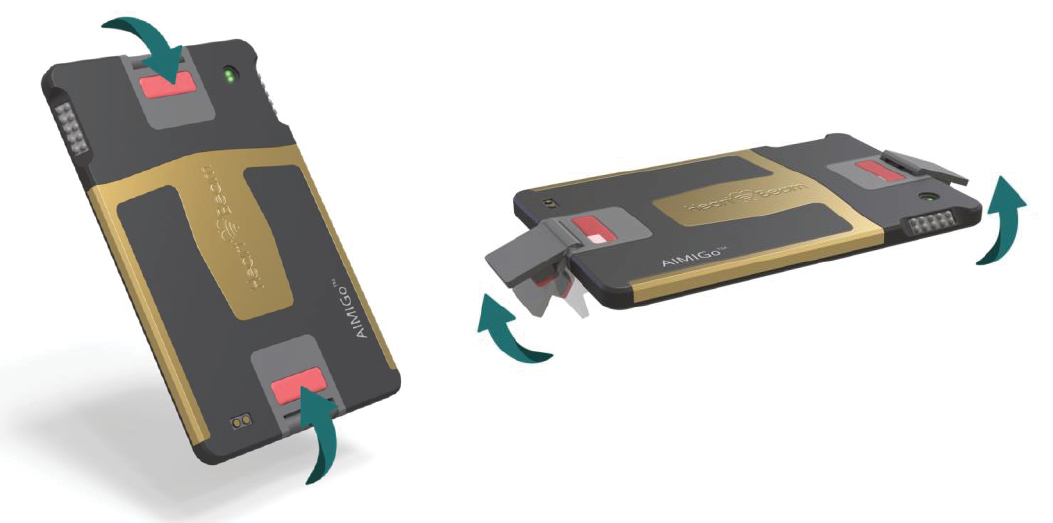
HeartBeam AIMIGo device in planar and ready position
We are also applying our software platform to create a tool for detecting heart attacks in the Emergency Department (“ED”) environment using standard 12-lead ECG recordings. The software tool, (“HeartBeam AIMITM”) is designed to enable emergency physicians diagnose heart attacks more accurately and quickly than currently available tools. Market clearance of this Product is planned to precede HeartBeam AIMIGo.
To date, we have developed working prototypes for both HeartBeam AIMIGo and HeartBeam AIMI. HeartBeam AIMI has been submitted for FDA 510(k) clearance and we have received questions from the FDA within the statutory 30-day review deadline, discussed the questions via teleconference with the FDA review team and provided written responses addressing the questions to the primary reviewer.
The custom software and hardware of our Products, we believe, are classified as Class II medical devices by the FDA, running on an FDA approved Class I registered software platform. Class II medical devices are those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish special controls. Special controls can include performance standards, post-market surveillance, patient histories and FDA guidance documents. Premarket review and clearance by the FDA for these devices is generally accomplished through the 510(k) or 510(k) de-novo premarket notification process.
HeartBeam has nine issued U.S. patents (U.S. 10,433,744, U.S. 10,117,592, U.S. 11,071,490, U.S. 11,419,538, U.S. 11,445,963, U.S. 11,529,085, U.S. 10,980,433, U.S. 11,412,972 and U.S. 11,234,658), and six pending U.S. applications. Four of the pending applications have been published, the remaining two pending cases are unpublished. Outside of the U.S., HeartBeam has four issued patents in Germany, France, Netherlands and United Kingdom and seven pending applications in Canada, China, the European Union, Japan and Australia. HeartBeam has three pending PCT applications. The issued patents are predicted to expire between April 11, 2036 and April 21, 2042.
Market Overview
Chronic diseases are the number one burden on the healthcare system, driving up costs each year, and cardiovascular illnesses are one of the top contributors. Regulators, payors, and providers are focused on shifting the diagnosis and management of these conditions to drive better outcomes at lower cost. Connected medical solutions are expanding rapidly and are projected to reach $155 billion by 2026, a compound annual growth rate (“CAGR”) of 17%. These solutions are socio-technical models for healthcare management and delivery using technology to provide healthcare services remotely and aim to maximize healthcare resources and provide increased, flexible capabilities for patients to engage with clinicians and better self-manage their care, using readily available consumer-facing technologies to deliver patient care outside the hospital or doctor’s office.
The market for Remote Patient Monitoring (“RPM”) is projected to reach $31.3 billion by 2023. In 2019, 1,800 hospitals in the US were using mobile applications to improve risk management and quality of care. The number in 2020 was likely larger as the onset of the COVID-19 pandemic greatly accelerated use and acceptance of telehealth by both patients and healthcare providers.
S-2
Cardiovascular disease is the number one cost to the healthcare system and is estimated to be responsible for 1 in every 6 healthcare dollars spent in the US. As cardiovascular disease is the leading cause of death worldwide, early detection, diagnosis, and management of chronic cardiac conditions are necessary to relieve the increasing burden on the healthcare infrastructure. Diagnostic tests such as ECGs are used to detect, diagnose, and track numerous cardiovascular conditions. With advances in mobile communications, diagnostic monitoring of cardiac conditions is increasingly occurring outside the hospital.
We intend to show that our easy to use device (without external electrodes) provides signals equivalent to a standard 12L device, and therefore will have a number of applications for ambulatory use. Our initial telemedicine technology Product HeartBeam AIMIGo will first address the heart attack detection market as well as the market to monitor CAD patients who are typically at high risk for a heart attack. Additionally, we expect to cater to patients across different risk profiles interested in our cardiac monitoring solutions for different heart conditions. Currently there are no products on the market that are user friendly, easy to carry, and always with the patient to provide physicians and patients with timely and highly accurate information about all heart conditions that could be detected with a 12L ECG, including potential ischemic events. A tool that is always with the patient, that decreases time to intervention, and that decreases the number of unnecessary ED visits by chest pain patients would have a significant effect on saving lives and healthcare dollars. We believe our technology will address this problem and will provide a convenient, cost-effective, integrated telehealth solution, including software and hardware for physicians and their patients. There are approximately 20 million people in the US who are considered at high risk for a heart attack, including 8 million who already have had prior intervention for MI’s and are therefore considered to be at extreme risk.
In the US, mobile cardiac tests are primarily conducted through outsourced Independent Diagnostic Testing Facilities (“IDTFs”) or as part of an RPM/telehealth system. Reimbursement rates vary depending on the use case and generally are based on the value a technology offers to patients and healthcare providers. Actual reimbursed pricing is set by the Centers for Medicare & Medicaid Services (“CMS”). Reimbursement rates for private insurers typically provide for similar or higher reimbursement rates when compared to those set by the government for Medicare and Medicaid.
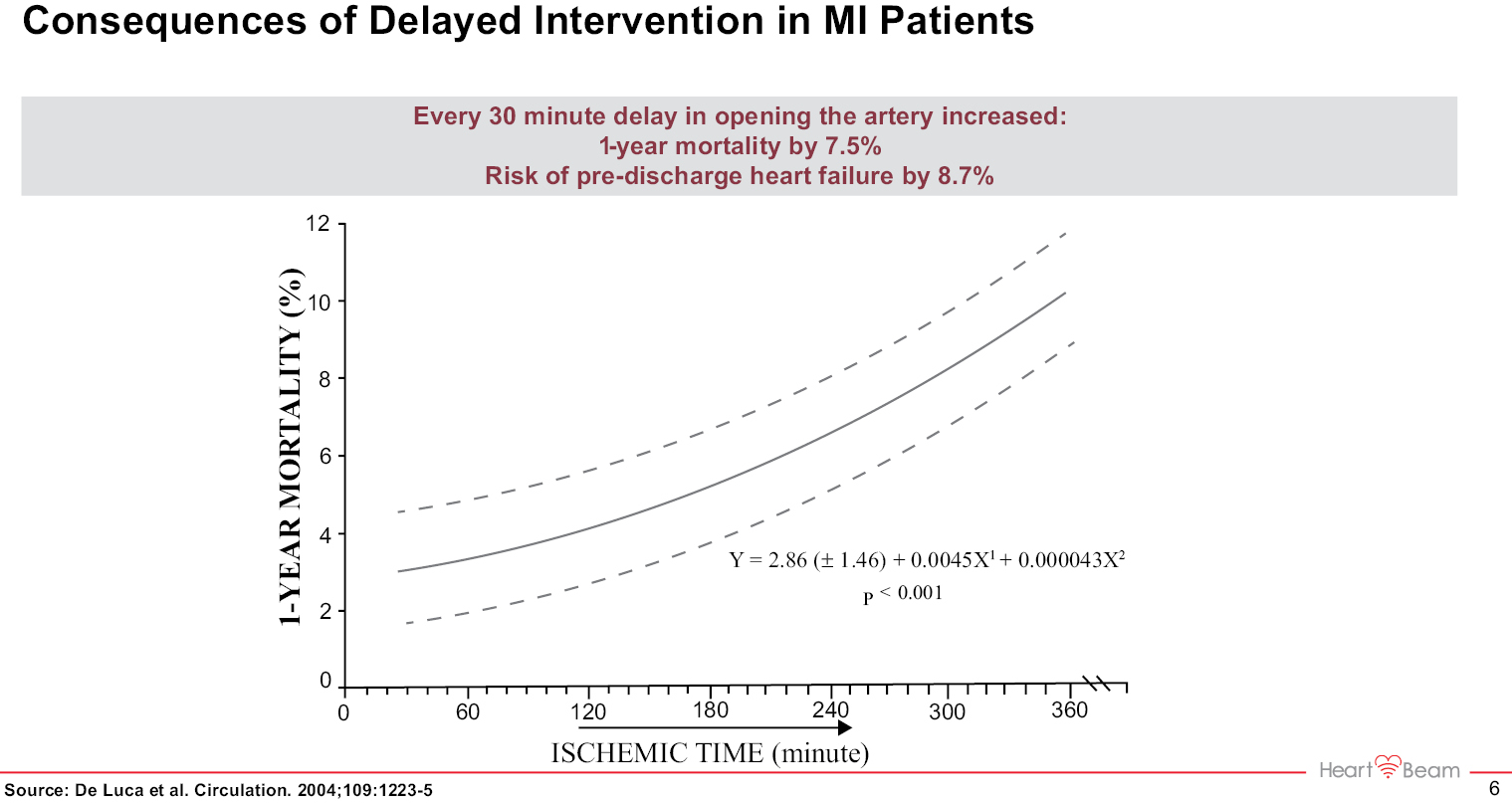
In the ED environment, early and accurate diagnosis of a chest pain patient who is potentially having a heart attack is of immense importance. Guidelines state that every chest pain patient in an ED must receive an ECG within 10 minutes of presentation. The accuracy of these initial ECGs, interpreted by physicians, is only approximately 75%. The need for increased ECG accuracy in detecting a heart attack in the ED is well defined, and an improved solution could result in saved lives and healthcare dollars. A 510(k) for our ED Product, HeartBeam AIMI, was submitted for review on August 15, 2022 to the FDA. We believe this Product will offer an increase in the accuracy of heart attack detection in EDs. There are approximately 5,000 Emergency Departments in the US.
S-3
Products and Technology
The foundation of our novel technology is the concept of VECG, a technology that has long been seen as superior to ECGs in detecting MIs but is no longer used clinically because of the difficulty experienced by physicians interpreting the output. We solved the crucial problem of recording three orthogonal (x, y and z) projections of the heart vector with a device that is sized like a credit card. The thickness of our credit card sized ECG signal collection device is about 1/8 inch (4 mm), and it weighs about 1 ounce (28 grams). The core technology consists of a series of patented inventions and associated algorithms. In addition to using VECG to get a more complete 3D characterization of cardiac activity, we use the concept of a baseline. Our MI marker is a differential marker that measures the change in cardiac parameters between an asymptomatic (baseline) recording and the symptomatic recording. It is personalized for every patient as every patient has a unique baseline. Our increase in diagnostic performance in detecting MIs, when compared to a panel of cardiologists, is attributed to a richer cardiac information set offered by VECG and the fact that our MI marker compares the baseline and symptomatic recordings and does that in the 3D space of VECG.
This novel technology has resulted in two key Products to date: a telehealth Product for cardiovascular patients (HeartBeam AIMIGo) and a powerful cloud-based ECG interpretation based on a quantitative comparison of the patients 3D VECG baseline and symptomatic recordings for EDs (HeartBeam AIMI). Our telehealth ECG collection device is the size of a credit card and records cardiac signals with integrated electrodes rather than wires or self-adhesive electrodes. Unlike a standard 12-lead ECG machine that records signals in empirically determined locations on a human body, our approach is focused on recording three projections of the heart vector. The successful recording of the projections of the heart vector enables the synthesis, via a patented method, of a 12-lead signal set and internal algorithmic diagnostic work in the space of 3D heart vectors.
There are obvious ease of use advantages when comparing our handheld device that fits in a wallet and can be instantly self-applied versus the current 12-lead ECG machine that requires a trained professional to apply. In addition, there are diagnostic performance advantages, including, based on our initial study, increased accuracy in diagnosing MIs. The system is used by patients at home or elsewhere, and collected data is sent to a physician to assess whether the patient’s chest pain is truly the result of an MI.
Our telehealth HeartBeam AIMIGo system will be a prescription-only mobile health system intended for individuals with known or suspected heart disease, especially CAD. It helps guide physicians in choosing the best course of action for their patients who experience chest pain outside of a medical facility. HeartBeam AIMIGo will bring a medical grade ECG to patients and will enable them to receive a plan of action from a physician in a timely manner. At the time of onboarding and at regularly scheduled time intervals, patients record a baseline 30 second cardiac reading using our device. When a patient experiences symptoms, such as irregular heartbeats or chest pain, the patient can simply open the smartphone application and press the credit card sized device against the chest to collect signals that can be converted to a synthesized 12-lead ECG. This synthesized 12-lead ECG is sent to the physician overlayed with the patient’s synthesized baseline ECG recording. In addition, the patient provides input on their symptoms that is sent, along with the ECG data, to the cloud for interpretation by a physician. A cloud-based algorithm processes the signals and displays the symptom description and patient history to a physician to analyze and prescribe an action plan. From start to finish, the process takes just a few minutes.
The telehealth HeartBeam AIMIGo system consists of a number of capabilities that will be productized in an incremental fashion. These are:
1. A credit card sized cardiac electrical signal collection device. The device captures cardiac signals that represent x, y and z projections of the heart vector and transmits them via Bluetooth connection to a smartphone. It is always with the patient as it easily fits in a wallet. It is easy to use as all that is required of the patient is that the device be pressed against the chest.
2. A smartphone application that receives the cardiac signals from the HeartBeam signal collection device. The application has several functions: guiding the patient through the signal collection, asking about symptoms, displaying the status of the data collection including real time signal quality check, and notifying the patient of the plan of action as determined by a physician. In addition, the application will contain HIPAA-compliant video conferencing or text capabilities for the healthcare provider to communicate directly with the patient.
S-4
3. A cloud-based software system that serves four basic functions: (1) Performing a final check of the ECG signal quality, (2) Synthesizing a 12-lead ECG from the measured (recorded) 3 vector leads, (3) Creating a diagnostic suggestion based on 3D VECG interpretation, risk factors and symptoms and (4) Preparing a summary report for the physician. These software functions will be introduced to the HeartBeam AIMIGo product in a sequential manner. To facilitate a more accurate physician interpretation of the data, the software overlays the patient’s synthesized baseline 12 lead ECG waveform on the synthesized 12 lead ECG waveform from the current event. To ensure high signal quality, the system checks for noise levels in the recorded signals. Those signals that can be effectively filtered are accepted and those that have a noise level above an empirically established threshold are rejected. If a recorded signal is rejected, the user is asked to repeat the recording.
4. A web-based physician portal capable of displaying the following relevant information for the physician to analyze: diagnostic suggestion, patient history, symptoms, baseline and current, synthesized 12-lead ECG, and recorded 3 vector leads. Our physician portal assists physicians with their diagnostic interpretation by providing both the baseline 12-lead synthesized ECG and the 12-lead synthesized ECG that is under evaluation.
5. A dedicated ECG monitoring and reading team of medical professionals to offer 24/7/365 services in order to assist symptomatic patients when making a decision of whether they should go to the Emergency Department. This capability will be developed in-house or outsourced through a contracted third-party organization.
The market release of our telehealth Product will be in multiple versions.
The initial Product will have a limited feature set and introduce a 3-lead 3D VECG credit card-sized device, the HeartBeam AIMIGo 3L, that records the X,Y,Z cardiac activity and displays the signals for clinician review, providing the regulatory foundation for subsequent products in our product portfolio. The 510(k) submission to the FDA is planned for early 2023.
Following this we will offer to the physician a pair of baseline and symptomatic 12-lead ECGs, both synthesized from 3-lead 3D VECG signals recorded by the HeartBeam AIMIGo device, and a symptoms report. It leverages recently issued patents for a personalized system for synthesizing 12-lead ECG waveforms. The 510(k) submission to the FDA is planned for late 2023.
Future versions may include our proprietary ECG interpretation MI marker and our overall MI diagnostic suggestion in addition to all features of the earlier Products and may as well offer an automated atrial fibrillation detection algorithm.
The same core technology built into the telehealth Product HeartBeam AIMIGo is used in the ED Product HeartBeam AIMI. In this application, the increased accuracy of detecting MIs by the ECG is of utmost importance. An ECG is the first diagnostic test a chest pain patient receives in the ED and it has a major impact on the patient’s subsequent clinical path. The ED Product has no hardware and introduces minimal change to the standard of care chest pain diagnostic path. It uses a baseline standard 12-lead ECG from the patient’s Electronic Medical Record (“EMR”) and the standard 12-lead ECG that is being evaluated. It converts the two ECGs to a VECG representation and utilizes our proprietary 3D VECG differential marker to generate an ECG interpretation suggestion to be used by the ED physician. The importance of increased accuracy of the first ECG interpretation for a chest pain patient presenting to an ED is significant, potentially leading to faster intervention or avoiding unnecessary activation of the cardiac catheterization lab.
FDA Regulatory Path
We have defined the FDA 510(k) clearance paths for both Products and have contracted with regulatory consultants to help us clear both products with the FDA.
The initial Product will have a limited feature set and introduce a 3-lead 3D VECG credit card-sized device, the HeartBeam AIMIGo 3L, that records the X, Y, Z cardiac activity and displays the signals for clinician review, providing the regulatory foundation for subsequent products in our product portfolio.
S-5
The 510(k) submission to the FDA is planned for early Q2 2023. We are planning a subsequent 510(k) submission, in Q4 2023, for the Product, which will include the ability to generate synthesized 12L ECG recordings. The submission is planned to contain results of a validation study comparing our synthesized 12-lead ECG recordings to standard 12-lead ECG recordings.
HeartBeam’s ED software product, HeartBeam AIMI, is hosted on LIVMOR’s Class I registered software platform and a predicate device for the cloud-based diagnostic engine for the ED product was identified. The predicate device is widely used as part of a software package produced by a leading ECG machine manufacturer. The predicate device software makes a diagnostic suggestion regarding a potential MI diagnosis. HeartBeam AIMI will also make a diagnostic suggestion to the ED physician. In the HIDES pilot study, we showed improved performance in detecting ischemia over a panel of cardiologists.
For the FDA 510(k) regulatory submission, a retrospective study was performed comparing patients’ baseline and asymptomatic ECG recordings and providing a diagnostic suggestion from the HeartBeam AIMI software. The diagnostic suggestion of the predicate device software, that was already recorded in the patient’s EMR, was compared to the diagnostic suggestion of our Product. The 510(k) regulatory submission for market clearance was filed with the FDA on August 15, 2022.
Market Opportunity
ECGs are key diagnostic tests utilized in the diagnosis and monitoring of cardiovascular disease, the number one cause of death worldwide. In the US in 2016, there were approximately 120 million adults living with cardiovascular disease and approximately 20 million adults with diagnosed coronary artery disease. The prevalence of these cardiac conditions and thus the market size is increasing, due to an aging population and lifestyle choices.
Every 40 seconds someone in the US has a heart attack, or MI. Unfortunately, there is no way for patients to tell whether the symptoms they are experiencing are due to an MI, or some other more benign condition such as indigestion. As a result, patients often ignore symptoms and delay seeking care, which leads to worse outcomes and increased mortality. On the other hand, many patients who go to the ED with chest pain are not experiencing an MI. Chest pain is the second most common reason for an ED visit in patients over 45, yet fewer than 20% of chest pain ED visits result in a diagnosis of a life-threatening condition. These unnecessary ED visits lead to well over $10 billion in unnecessary healthcare expenditures.
Most ECGs are conducted in a healthcare facility setting using a 12-lead ECG machine, the gold standard. ECGs taken outside of healthcare facilities are expected to grow more quickly than in-hospital ECGs. Monitoring cardiac patients outside of a hospital is a fast-growing trend, as it is less expensive and provides a better patient experience. However, while ambulatory cardiac monitoring devices are often much easier for patients to use, they have fewer leads than the gold standard and therefore cannot offer as comprehensive a picture of cardiac health.
While a standard 12-lead ECG readout is of great medical value, it is simply impractical to have a standard 12-lead machine next to patients when they experience symptoms outside the clinical setting, since recording the event requires attaching multiple electrodes to the patient’s body with professional assistance. While existing technologies use predominantly single lead ECG devices to monitor arrhythmias, these technologies do not provide information to the physician on the presence of life-threatening conditions of acute coronary syndrome (“ACS”) or heart attacks.
We believe our telehealth technology addresses these market needs and has several key attributes that make it a good fit for these patients. Our telehealth Product is generally used when symptoms occur and offers the potential for lifelong patient usage. The device is practically always near the patient and ready to be used for recording a cardiac event. It enables very nearly real-time cardiac data transmission during a telemedicine visit. It offers a recorded 3 vector lead set of signals and a synthesized 12-lead ECG set of signals. We believe physicians will typically prescribe our solution to chronic cardiovascular patients for long term monitoring, thereby enabling prolonged data collection and delivering a more complete picture for diagnosis. This will also enable the use of artificial intelligence on our future database that will have a unique set of longitudinal synthesized 12-lead ECGs for patients.
As our VECG platform demonstrates 12-Lead equivalence and clinical & cost-effectiveness advantages, coupled with a patent protected technology, we believe this might open multiple licensing and/or partnering opportunities with players in the ECG, cardiac monitoring patch and smart watch verticals.
S-6
Market Strategy
Our goal is to establish our products as key solutions for cardiology practices and hospital EDs. Our efforts to enter the market involve establishing clinical evidence and demonstrating the cost-effectiveness of adopting our products. For both the telehealth (HeartBeam AIMIGo) and the ED Products (HeartBeam AIMI), the initial geographic market is the United States.
We believe that both the telehealth and ED Products will be subject to the US FDA’s 510(k) review process. A 510(k) for our HeartBeam AIMI was submitted to the FDA on August 15, 2022, for review and we are in the process of preparing a 510(k) submission for HeartBeam AIMIGo.
For HeartBeam AIMIGo, the primary customers are cardiology practices and the cardiology departments of hospitals. Healthcare insurers are another important customer, as they will potentially benefit from the reduced costs to the healthcare system. We are working to develop new clinical studies and publish results of completed clinical studies and plan to demonstrate real world cost-effectiveness of the use of the solution.
Our initial targets for HeartBeam AIMIGo are market segments that see value in an easy-to-use device that can generate synthesized 12L ECG recordings. These will be segments in which payment for the device will be outside of the established reimbursement system. These target segments include concierge practices, hospital-at-home segment and clinical trials. As we establish clinical data on the clinical and cost-effectiveness of HeartBeam AIMIGo, we will target at-risk cardiology practices, including high risk patients being discharged from hospitals after experiencing an MI.
Our long-term strategy is to generate sufficient evidence of clinical efficacy and cost-effectiveness to generate reimbursement coverage and payment specifically for the HeartBeam AIMIGo solution. We expect to be able to demonstrate significant clinical benefits for patients and savings to the healthcare system, justifying appropriate reimbursement levels.
For the telehealth Product, our primary marketing strategy will focus on the medical community with continued validation of clinical efficacy and cost-effectiveness and the establishment of reference sites. We will also create educational materials and provide other support to help educate our customers’ patients.
We will explore other business models. For example, hospitals face CMS penalties if their 30-day readmission rates for patients who are discharged after an MI exceed certain thresholds. These CMS penalties are levied on all hospital CMS payments, so the impact can be significant. Our telehealth Product can be a tool to help hospitals manage these patients after discharge. We will explore models in which hospitals pay for the device and for the initial 30 days of service. In addition, we will explore models for value-based care, in which the use of the telehealth Product reduces overall costs.
We are currently speaking with hospitals in large healthcare systems to educate them about our first two products. These are sophisticated customers, and we plan to use technical presentations, peer reviewed clinical data, and demonstration projects to achieve penetration of this market. We plan to continue to expand our medical advisory board, conduct clinical trials with leading cardiologists to increase the body of evidence, and establish reference sites among these customers.
For the ED Product, the primary customers are acute care facilities. As with the telehealth Product, we plan to publish clinical studies on the effectiveness of the Product. In addition, we plan to develop financial models demonstrating the cost-effectiveness of the approach and establish reference sites who are using the Product. We do not expect to obtain specific reimbursement for the ED Product but intend to demonstrate that the purchase of the software would result in clinical and economic benefits and potentially reduced malpractice legal exposure for ED provider institutions.
We expect our value proposition will be progressively increased as we gradually add additional functionality to our monitoring solutions and drive down the cost of continuous monitoring by increasing scale and automation. We expect our HeartBeam AIMIGo device and AIMI software to gradually incorporate internally developed algorithms with the capabilities of detecting heart conditions that can be exposed via a standard 12-L ECG device. Additionally, as we collect rich longitudinal data sets from our patients, we expect to train AI and ML algorithms that could potentially have predictive capabilities regarding different heart conditions. Over time and with scale we expect our costs to decrease and provide more and better services to our patients by improving our capabilities.
We plan to establish a direct sales network with relationships and experience selling to our target markets.
S-7
Clinical Data
HeartBeam has performed three initial clinical studies to assess performance of our technologies.
In the first study (HeartBeam Ischemia Detection Study — HIDES), we simultaneously collected traditional 12-lead ECG and vector X,Y and Z signals on patients whose coronary arteries were occluded during a Percutaneous Coronary Intervention (PCI). Our VECG-based signal interpretation system had significantly (21%) higher accuracy in detecting ischemia.
In a second study (B Score), the HeartBeam diagnostic engine, using ECG, symptoms, and history, matched the diagnostic performance of expert cardiologists in detecting the presence of MIs in patients presenting to an ED with chest pain. This result indicates that the quality of the diagnostic advice produced by our expert system will be extremely valuable to the physician who is assessing the condition of a patient in a telehealth environment.
The third study, ISPEC, which assessed the false positive rate for non-symptomatic patients, is relevant in a telehealth situation. It is important that the system have a low false positive rate when patients are conducting baseline recordings, which are required on at least a monthly basis. The study yielded no false positives.
All three studies are being prepared for peer-reviewed publication.
Intellectual Property
We believe our intellectual property (“IP”) protects our innovations, and our goal is to become a leader in the ambulatory VECG sector. For some aspects of our proprietary technology, we rely on trade secret protection. It is our view that the combination of these two methods of IP protection maximizes our chances for success.
HeartBeam has nine issued U.S. patents (U.S. 10,433,744, U.S. 10,117,592, U.S. 11,071,490, U.S. 11,419,538, U.S. 11,445,963, U.S. 11,529,085, U.S. 10,980,433, U.S. 11,412,972 and U.S. 11,234,658), and six pending U.S applications. Four of the pending applications have been published, the remaining two pending cases are unpublished. Outside of the U.S., HeartBeam has four issued patents in Germany, France, Netherlands, and United Kingdom and seven pending applications in Canada, China, the European Union (“EU”), Japan and Australia. HeartBeam has three pending PCT applications. The issued patents are predicted to expire between April 11, 2036 and April 21, 2042.
Our issued and pending U.S. patent applications cover compact VECG systems for remote detection and/or diagnosis of acute myocardial infarction (“AMI”). Outside of the U.S., the pending EU, Australian (“AU”), Japanese (“JP”) and Chinese (“CN”) patent applications correspond to the pending and issued US cases. The pending PCT applications cover methods and apparatuses for automatic cardiac diagnosis as well as compact systems including retractable electrodes.
Research and Development
The primary objective of our research and development program is to provide innovative, user-friendly, ambulatory VECG solutions with high medical value. To date, we have been highly successful in developing our initial products. The emphasis has been on developing a user-friendly solution that is always with the patient and assists physicians in diagnosing heart attacks in chest pain patients.
Our Research team is largely based in Belgrade, Serbia as well as in California, USA. We have assembled a highly capable Belgrade team currently consisting of seven PhD level contributors who are credited with developing our key inventions and patents. The diverse group includes:
• Two nuclear physicists.
• Two Biomedical Engineers experienced in developed digital health applications.
• Two highly experienced Healthcare IT development professionals.
• Two Electrical Engineers (M.S.E.E) with strong signal processing and ECG analysis algorithm expertise from the medical device industry.
S-8
• An Electrical Engineer (M.S.E.E) with exceptional implantable medical device development and power optimization expertise.
• A Software Engineer, (PhD Computer Science), with deep expertise in developing mobile applications for medical devices.
Future research and development efforts will focus on the application of signal processing and artificial intelligence to address a range of cardiac conditions.
During 2022 we also added 3 development engineers led by our recently hired Chief Technology Officer Kenneth Persen. This is an experienced development team that worked together previously and was successful in delivering FDA cleared products.
Future Products
Our core technology — the heart vector approach adopted and invented by our research team — is a platform technology that can provide diagnostic solutions to a variety of cardiovascular patients. Our plans call for expanding solutions that diagnose all major cardiac conditions that are diagnosed by ECGs.
Our future plans include the development of a VECG-based, synthesized 12-lead capable patch ECG monitor that will provide advantages over existing single-lead ECG patch products such as the Zio-XT from iRhythm Technologies, Inc. Our approach will offer a synthesized 12-lead ECG with a patch that is very similar, in dimensions and look and feel, to the currently available single lead ECG patches. We believe providing standard of care 12-lead ECG capabilities will have significant diagnostic advantages over a single lead patch.
We also plan to integrate a synthesized 12-lead ECG smartwatch-based monitor intended for detection of heart attacks and complex cardiac arrhythmias. This invention eliminates the need for a dedicated ECG device while offering a synthesized 12-lead ECG capability enabling heart attack and complex arrhythmia detection.
While our initial telehealth Product is powered by a software expert system that serves as a diagnostic aid to a physician, we will develop an AI based diagnostic system that will supplement our diagnostic expert system.
We are committed to continue advancing the full potential inherent in our synthesized 12-lead 3D VECG technology as demonstrated in recently issued and allowed patents with potentially disruptive market impacts.
Corporate Information
HeartBeam was incorporated as a C corporation under the laws of the State of Delaware on June 11, 2015. We do not own any subsidiaries. Our corporate offices are located at 2118 Walsh Avenue, Suite 210, Santa Clara, CA 95050. Our telephone number is (408) 899-4443. The address of our website is https://www.heartbeam/. The inclusion of our website address in this prospectus does not include or incorporate by reference the information on our website into this prospectus.
S-9
OFFERING SUMMARY
This summary highlights certain information about this offering and selected information contained elsewhere in or incorporated by reference into this prospectus supplement. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in securities. For a more complete understanding of our company and this offering, we encourage you to read and consider carefully the more detailed information in this prospectus supplement and the accompanying base prospectus, including the information incorporated by reference into this prospectus supplement and the accompanying base prospectus, and the information referred to under the heading “RISK FACTORS” in this prospectus supplement on page S-11 and on page 9 of the accompanying base prospectus, and in the documents incorporated by reference into this prospectus supplement and the accompanying base prospectus.
|
Issuer |
HeartBeam, Inc. |
|
|
Common stock offered by us |
1,000,000 shares |
|
|
Common stock outstanding prior to the offering(1) |
|
|
|
Common stock to be outstanding after this offering(1) |
|
|
|
Common Stock Trading symbol |
Our Common Stock is traded on Nasdaq Capital Market under the symbol “BEAT”. |
|
|
Use of proceeds |
We intend to use the net proceeds from the offering for working capital and general corporate purposes. See “Use of Proceeds.” |
|
|
Risk factors |
This investment involves a high degree of risk. See “Risk Factors” and other information included or incorporated by reference in this prospectus supplement beginning on page S-11 and the accompanying base prospectus beginning on page 9 for a discussion of certain factors you should carefully consider before deciding to invest in shares of our common stock. |
____________
(1) The number of shares of Common Stock outstanding is based on shares of Common Stock issued and outstanding as of December 31, 2022 plus the 16,666,666 shares of Common Stock issued on May 2, 2023 and excludes the following:
• 2,196,798 shares of Common Stock issuable upon the exercise of outstanding stock options having a weighted average exercise price of $1.76 per share;
• 253,970 shares of Common Stock issuable upon vesting of RSUs;
• 3,908,276 shares of Common Stock issuable upon the exercise of outstanding warrants having a weighted average exercise price of $5.42 per share;
• 747,364 shares of Common Stock reserved for future issuance under the Company’s 2022 Equity Incentive Plan.
Except as otherwise indicated herein, all information in this prospectus reflects or assumes:
• no exercise of the outstanding options described above;
S-10
Investment in any securities offered pursuant to this prospectus and the applicable prospectus supplement involves risks. You should carefully consider the risk factors incorporated by reference to our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q or Current Reports on Form 8-K we file after the date of this prospectus, and all other information contained or incorporated by reference into this prospectus, as updated by our subsequent filings under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the risk factors and other information contained in the applicable prospectus supplement before acquiring any of such securities. The occurrence of any of these risks might cause you to lose all or part of your investment in the offered securities.
Risks Relating to this Offering
We may allocate the net proceeds from this offering in ways that you or other stockholders may not approve.
We currently intend to use the net proceeds of this offering, if any, for working capital and general corporate purposes, which may include capital expenditures, research and development expenditures, regulatory affairs expenditures, clinical trial expenditures, acquisitions of new technologies and investments, and the financing of possible acquisitions or business expansions. This expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development efforts, the status of and results from clinical trials, as well as any third-party intellectual property or other assets that we may opportunistically identify and seek to license or acquire or any collaborations that we may enter into with third parties for our product candidates, and any unforeseen cash needs. Because the number and variability of factors that will determine our use of the proceeds from this offering, their ultimate use may vary substantially from their currently intended use. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering and could spend the proceeds in ways that do not necessarily improve our operating results or enhance the value of our common stock. See “Use of Proceeds.
Sales of our common stock in this offering, or the perception that such sales may occur, could cause a drop in the market price of our common stock.
We may issue and sell shares of our common stock for aggregate gross proceeds of up to $13 million from time to time in connection with this offering. The issuance and sale from time to time of these new shares of common stock, or our ability to issue these new shares of common stock in this offering could have the effect of depressing the market price of our common stock.
We may sell additional shares of our common stock to fund our operations, which sales may occur during or immediately after sales pursuant to this offering are commenced, which would result in dilution to our shareholders.
In order to raise additional funds to support our operations, we may sell additional shares of our common stock, which would result in dilution to all of our shareholders that may adversely impact our business. See “Dilution.” In particular, at any time, including during the pendency of this offering, we may sell additional shares of our common stock, other than pursuant to this offering, in amounts that may be material to us, which may be in amounts that are equal to or greater than the size of this offering, including, without limitation, through underwritten public offerings, privately negotiated transactions, block trades, or any combination of the above, subject, in certain circumstances, to the consent of the Agent. We cannot assure you that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing shareholders. The price per share at which we sell additional shares of our common stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher or lower than the price per share in this offering.
S-11
SPECIAL NOTICE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve risks and uncertainties, principally in the sections entitled “Risk Factors.” All statements other than statements of historical fact contained in this prospectus, including statements regarding future events, our future financial performance, business strategy and plans and objectives of management for future operations, are forward-looking statements. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” or “will” or the negative of these terms or other comparable terminology. Although we do not make forward looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks outlined under “Risk Factors” or elsewhere in this prospectus, which may cause our or our industry’s actual results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Forward-looking statements should not be read as a guarantee of future performance or results, and will not necessarily be accurate indications of the times at, or by which, that performance or those results will be achieved. Forward-looking statements are based on information available at the time they are made and/or management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from what is expressed in or suggested by the forward-looking statements.
Forward-looking statements speak only as of the date they are made. You should not put undue reliance on any forward-looking statements. We assume no obligation to update forward-looking statements to reflect actual results, changes in assumptions or changes in other factors affecting forward-looking information, except to the extent required by applicable securities laws. If we do update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
S-12
We currently intend to use the net proceeds from this offering, if any, for working capital and general corporate purposes.
The timing and amount of our actual expenditures will be based on many factors, including cash flows from operations and the anticipated growth of our business. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering. As a result, our management will have broad discretion regarding the timing and application of the net proceeds from this offering. Pending their ultimate use, we intend to invest the net proceeds in short-term, investment-grade, interest-bearing instruments.
MARKET PRICE OF OUR COMMON STOCK
Our common stock is presently listed on The NASDAQ Global Market under the symbol “BEAT”. On May 3 2023, the last reported sale price of our common stock was $2.31.
Holders
As of May 3, 2023 we had 50 registered holders of record of our common stock. A substantially greater number of holders of our common stock are “street name” or beneficial holders, whose shares of record are held through banks, brokers, other financial institutions and registered clearing agencies.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain our future earnings, if any, for use in our business and therefore do not anticipate paying cash dividends in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs and plans for expansion.
DESCRIPTION OF SECURITIES WE ARE OFFERING
In this offering, we are offering a 1,000,000 maximum of shares of our Common at public offering price of $1.50 per share.
The material terms and provisions of our Common Stock are described under the caption “Description of Capital Stock” starting on page 12 of the accompanying base prospectus.
Transfer Agent
The transfer agent and registrar for our common stock is VStock Transfer, LLC with an address at 18 Lafayette Pl, Woodmere, NY 11598.
S-13
The validity of the shares of common stock offered hereby will be passed upon for us by Lucosky Brookman LLP.
The financial statements as of and for the year ended December 31, 2022 incorporated by reference in this registration statement have been audited by Marcum LLP, an independent registered public accounting firm, as stated in their report (the report on the financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern). Such financial statements are incorporated by reference in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
The financial statements as of and for the year ended December 31, 2021 incorporated by reference in this registration statement have been audited by Friedman LLP, an independent registered public accounting firm, as stated in their report. Such financial statements are incorporated by reference in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
S-14
The SEC’s rules allow us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and subsequent information that we file with the SEC will automatically update and supersede that information. Any statement contained in a previously filed document incorporated by reference will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus modifies or replaces that statement.
We incorporate by reference our documents listed below and any future filings made by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, which we refer to as the “Exchange Act” in this prospectus, between the date of this prospectus and the termination of the offering of the securities described in this prospectus. We are not, however, incorporating by reference any documents or portions thereof, whether specifically listed below or filed in the future, that are not deemed “filed” with the SEC, including any information furnished pursuant to Items 2.02 or 7.01 of Form 8-K or related exhibits furnished pursuant to Item 9.01 of Form 8-K.
This prospectus supplement incorporates by reference the documents listed below, other than those documents or the portions of those documents deemed to be furnished and not filed in accordance with SEC rules:
• our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on March 16, 2023;
• our Current Reports on Form 8-K filed with the SEC on January 24, 2023, February 2, 2023, March 3, 2023, March 8, 2023, March 9, 2023, March 13, 2023, March 24, 2023, and May 3, 2023; and
• our 2022 equity incentive plan on Form S-8 filed with the SEC July 13, 2022;
All reports and other documents we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of this offering, including all such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated by reference into this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
You may request a free copy of any of the documents incorporated by reference in this prospectus (other than exhibits, unless they are specifically incorporated by reference in the documents) by writing or telephoning us at the following address:
Branislav Vajdic
Chief Executive Officer
2118 Walsh Avenue, Suite 210
Santa Clara, CA 95050
Telephone: 408-899-4443
Exhibits to the filings will not be sent, however, unless those exhibits have specifically been incorporated by reference in this prospectus and any accompanying prospectus supplement.
S-15
PROSPECTUS
HeartBeam, Inc.
$100,000,000
Common Stock
Preferred Stock
Debt Securities
Warrants
Rights
Units
We may offer and sell up to $100,000,000 in the aggregate of the securities identified above from time to time in one or more offerings. This prospectus provides you with a general description of the securities.
Each time we offer and sell securities, we will provide a supplement to this prospectus that contains specific information about the offering and the amounts, prices and terms of the securities. The supplement may also add, update or change information contained in this prospectus with respect to that offering. You should carefully read this prospectus and the applicable prospectus supplement before you invest in any of our securities.
We may offer and sell the securities described in this prospectus and any prospectus supplement to or through one or more underwriters, dealers and agents, or directly to purchasers, or through a combination of these methods. If any underwriters, dealers or agents are involved in the sale of any of the securities, their names and any applicable purchase price, fee, commission or discount arrangement between or among them will be set forth, or will be calculable from the information set forth, in the applicable prospectus supplement. See the sections of this prospectus entitled “About this Prospectus” and “Plan of Distribution” for more information. No securities may be sold without delivery of this prospectus and the applicable prospectus supplement describing the method and terms of the offering of such securities.
INVESTING IN OUR SECURITIES INVOLVES RISKS. SEE THE “RISK FACTORS” ON PAGE 9 OF THIS PROSPECTUS AND ANY SIMILAR SECTION CONTAINED IN THE APPLICABLE PROSPECTUS SUPPLEMENT CONCERNING FACTORS YOU SHOULD CONSIDER BEFORE INVESTING IN OUR SECURITIES.
Our common stock and warrants are listed on The NASDAQ Capital Market under the symbol “BEAT” and “BEATW”, respectively. On March 7, 2023, the last reported sale price of our common stock on The NASDAQ Capital Market was $3.39 per share.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is February 10, 2023.
TABLE OF CONTENTS
|
Page |
||
|
ii |
||
|
1 |
||
|
9 |
||
|
10 |
||
|
11 |
||
|
12 |
||
|
16 |
||
|
22 |
||
|
24 |
||
|
25 |
||
|
26 |
||
|
28 |
||
|
28 |
||
|
28 |
||
|
29 |
i
This prospectus is part of a registration statement that we filed with the U.S. Securities and Exchange Commission (the “SEC”), using a “shelf” registration process. By using a shelf registration statement, we may sell securities from time to time and in one or more offerings up to a total dollar amount of $100,000,000 as described in this prospectus. Each time that we offer and sell securities, we will provide a prospectus supplement to this prospectus that contains specific information about the securities being offered and sold and the specific terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus with respect to that offering. If there is any inconsistency between the information in this prospectus and the applicable prospectus supplement, you should rely on the prospectus supplement. Before purchasing any securities, you should carefully read both this prospectus and the applicable prospectus supplement, together with the additional information described under the headings “Where You Can Find More Information” and “Incorporation by Reference.”
We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We will not make an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus and the applicable prospectus supplement to this prospectus is accurate as of the date on its respective cover, and that any information incorporated by reference is accurate only as of the date of the document incorporated by reference, unless we indicate otherwise. Our business, financial condition, results of operations and prospects may have changed since those dates.
When we refer to “BEAT,” “we,” “our,” “us” and the “Company” in this prospectus, we mean HeartBeam, Inc., unless otherwise specified. When we refer to “you,” we mean the holders of the applicable series of securities.
ii
Corporate History and Information
HeartBeam was incorporated as a C corporation under the laws of the State of Delaware on June 11, 2015. We do not own any subsidiaries.
Company Overview
We are a medical technology company primarily focusing on developing and commercializing higher resolution ambulatory Electrocardiogram (“ECG”) solutions that enable the detection and monitoring of cardiac disease outside a healthcare facility setting. Our ability to develop higher resolution ECG solutions is achieved through the development of our proprietary and patented Vector Electrocardiography (“VECG”) technology platform. Our vector electrocardiography technology is capable of developing three-dimensional (3D) images of cardiac electrical activity by displaying the spatial locations of ECG waveforms that has demonstrated in early studies to deliver equal or superior diagnostic capability than traditional hospital based ECG systems.

3D projections of cardiac vectors
Our aim is to deliver innovative, remote patient monitoring (“RPM”) technologies that can be used for patients anywhere where critical cardiac care decisions can be made on a more timely basis. Our products require Food and Drug Administration (“FDA”) clearance and have not been cleared for marketing (hereinafter “Product” or “Products”.)
We believe our Products and services will benefit many stakeholders, including patients, healthcare providers, and healthcare payors. We are developing Generation 1 of our telehealth product (“HeartBeam AIMIGoTM”) to address the rapidly growing field of RPM. HeartBeam AIMIGo is comprised of a credit card sized electrocardiogram device and a powerful cloud-based diagnostic expert software system. We believe that we are uniquely positioned to play a central role in remote monitoring of high-risk coronary artery disease patients, because the initial studies have shown that our ischemia detection system may be more accurate than existing RPM solutions.

Photographs of version 1 HeartBeam AIMIGo devices in planer and ready to use position.
1
We are also applying our software platform to create a tool for detecting heart attacks in the emergency room environment using traditional ECG devices. The software tool, (“HeartBeam AIMI(TM)”) is designed to enable emergency physicians to more accurately and quickly diagnose heart attacks than currently available. Market release of this Product will precede that of HeartBeam AIMIGo.
To date, we have developed working prototypes for both HeartBeam AIMIGo and HeartBeam AIMI. HeartBeam AIMI has been submitted for FDA 510(k) clearance and we have received questions from the FDA within the statutory 30-day review deadline, discussed the questions via teleconference with the FDA review team and provided written responses addressing the questions to the primary reviewer. We believe we are on track for FDA clearance this quarter.
The custom software and hardware of our Products, we believe, are classified as Class II medical devices by the FDA, running on an FDA approved Class I registered platform. Class II medical devices are those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish special controls. Special controls can include performance standards, post-market surveillance, patient histories and FDA guidance documents. Premarket review and clearance by the FDA for these devices is generally accomplished through the 510(k) or 510(k) de-novo premarket notification process.
Recent Developments not Incorporated by Reference
In September 2022, we were granted two patents;
• We were granted a 12-lead ECG patch monitor intended for detection of acute coronary syndrome (“ACS”) and cardiac arrhythmia by the United States Patent and Trademark Office. The innovation builds on our growing intellectual property portfolio enabling synthesized 12-lead ECG diagnostics outside of a medical setting.
• We were also granted a patent that enables generation of a synthesized 12-lead ECG by the HeartBeam AIMIGo credit card-sized device by the United States Patent and Trademark Office. The innovation opens the pathway for a patient to record a set of signals using HeartBeam AIMIGo outside of a medical setting with a diagnostic synthesized 12-lead ECG immediately transmitted to a physician for review and diagnosis. Unlike single-lead ECG products currently in the marketplace, such as other credit card sized devices or smartwatches, our technology is intended to quickly and accurately help a physician identify a heart attack (“Myocardial Infarction” or “MI”).
In October 2022, we announced the expansion of our product pipeline with smartwatch connectivity enablement for 24/7 heart monitoring capability. The product pipeline advancement allows for the addition of arrhythmia detection capabilities to address the multibillion-dollar global market for atrial fibrillation and other arrhythmia monitoring. This capability builds on our recently issued patents. This broader product portfolio enables the following:
• Introducing a 3-lead 3D vector electrocardiogram credit card-sized device, the HeartBeam AIMIGo 3L, that records the X,Y,Z cardiac activity and displays the signals for clinician review, providing the regulatory foundation for subsequent products in our product portfolio. The 510(K) submission to the FDA is planned for Q4 2022.
• Leveraging recently issued patents to incorporate both synthesized baseline and symptomatic 12-lead signals for enhanced diagnostic accuracy as well as the addition of atrial fibrillation detection capability in the HeartBeam AIMIGo 12L device for FDA 510(K) submission in Q2 2023.
• Broadening of the product portfolio profile to enable smartwatch connectivity to our platform in future products as an optional monitoring solution for the clinician and the patient.
In October 2022, we announced the appointment of Peter J. Fitzgerald, MD, Ph. D, as Chief Medical Officer. Dr. Fitzgerald is the Director of the Center for Cardiovascular Technology and Director of the Cardiovascular Core Analysis Laboratory at Stanford University Medical School. In addition to his world-renowned expertise in interventional cardiology, Dr. Fitzgerald is an accomplished inventor, entrepreneur, and investment fund founder.
In November 2022, we announced that our patent for a 12-lead ECG smartwatch-based monitor intended for detection of heart attacks and complex cardiac arrhythmias was allowed by the United States Patent and Trademark Office. The innovation builds on our growing intellectual property portfolio enabling 12-lead ECG diagnostics outside of a medical setting.
2
Market Overview
Chronic diseases are the number one burden on the healthcare system, driving up costs each year, and cardiovascular illnesses are one of the top contributors. Regulators, payors and providers are focused on shifting the diagnosis and management of these conditions to drive better outcomes at lower cost. Connected medical solutions are expanding rapidly and are projected to reach $155 billion by 2026, a compound annual growth rate (“CAGR”) of 17%. These solutions are socio-technical models for healthcare management and delivery using technology to provide healthcare services remotely and aim to maximize healthcare resources and provide increased, flexible opportunities for consumers to engage with clinicians and better self-manage their care, using readily available consumer technologies to deliver patient care outside of the hospital or doctor’s office. The types of companies that make up this market include Accenture, IBM, SAP, GE Healthcare, Oracle, Microsoft, Airstrip Technology, Medtronic, Allscripts, Boston Scientific, Athenahealth, Cerner, Philips, Agamatrix, Qualcomm, and AliveCor.
The market for RPM, is projected to reach $31.3 billion by the end of 2023. In 2019, 1,800 hospitals in the US were using mobile applications to improve risk management and quality of care. The number in 2020 was likely larger as the onset of the COVID-19 pandemic greatly accelerated use and acceptance of telehealth by both patients and healthcare providers.
Cardiovascular disease is the number one cost to the healthcare system and is estimated to be responsible for 1 in every 6 healthcare dollars spent in the US. As cardiovascular disease is the leading cause of death worldwide, early detection, diagnosis, and management of chronic cardiac conditions are necessary to relieve the increasing burden on the healthcare infrastructure. Diagnostic tests such as ECGs are used to detect, diagnose and track numerous cardiovascular conditions. With advances in mobile communications, diagnostic monitoring of cardiac conditions is increasingly occurring outside the hospital.
Our initial telemedicine technology Product will address the heart attack detection market as well as the market to monitor coronary artery disease (“CAD”) patients who are typically at high risk for a heart attack. Currently there are no products on the market that are user friendly, easy to carry, and always with the patient in order to provide physicians and patients with timely and highly accurate information about potential ACS and MI events. A tool that is always with the patient, that decreases time to intervention, and that decreases the number of unnecessary ED visits by chest pain patients would have a significant effect on saving lives and healthcare dollars. We believe our technology will address this problem and will provide a convenient, cost-effective, integrated telehealth solution, including software and hardware for physicians and their patients. There are approximately 18 million people in the US who are considered at high risk for a heart attack, including 8 million who already have had prior intervention for MI’s and are therefore considered to be at extreme risk.
In the US, mobile cardiac tests are primarily conducted through outsourced Independent Diagnostic Testing Facilities (“IDTFs”) or as part of an RPM system. Reimbursement rates vary depending on the use case and generally are based on the value a technology offers to patients and healthcare providers. Actual reimbursed pricing is set by the Centers for Medicare & Medicaid Services (“CMS”). Reimbursement rates for private insurers typically provide for similar or better reimbursement rates when compared to those set by the Government for Medicare and Medicaid.
In the ED environment, early and accurate diagnosis of a chest pain patient who is potentially having a heart attack is of immense importance. Guidelines state that every chest pain patient in an ED must receive an ECG within 10 minutes of presentation. The accuracy of these initial ECGs is only approximately 75%. The need for increased ECG accuracy in detecting a heart attack in the ED is well defined, and an improved solution could result in saved lives and healthcare dollars. A 510(K) for our ED Product was submitted for review on August 15, 2022 to the FDA. We believe this Product will offer a marked increase in the accuracy of heart attack detection in EDs. There are approximately 5,000 ED departments in the US.
Products and Technology
The foundation of our novel technology is the concept of VECG, a technology that has long been seen as superior to ECGs in detecting MIs but is no longer used clinically because of the difficulty experienced by physicians interpreting the output. We solved the crucial problem of recording three orthogonal (x, y and z) projections of the heart vector with a device that is sized like a credit card. The thickness of our credit card sized ECG signal collection device is about 1/8 inch (3 mm), and it weighs about 1 ounce (28 grams). The core technology consists of a series of patented inventions and associated algorithms. In addition to using VECG to get a more complete 3D characterization of
3
cardiac activity, we use the concept of a baseline. Our MI marker is a differential marker that measures the change in cardiac parameters between an asymptomatic (baseline) recording and the symptomatic recording. It is personalized for every patient as every patient has a unique baseline. Our increase in diagnostic performance in detecting MIs, when compared to a panel of cardiologists, is attributed to a richer cardiac information set offered by VECG and the fact that our MI marker compares the baseline and symptomatic recordings and does that in the 3D space of VECG.
This novel technology has resulted in two key Products to date: a telehealth Product for high-risk cardiovascular patients (HeartBeam AIMIGo) and a powerful cloud-based diagnostic expert and MI detection system for EDs (HeartBeam AIMI). Our telehealth ECG collection device is the size of a credit card and records cardiac signals with integrated electrodes rather than wires or self-adhesive electrodes. Unlike a standard 12-lead ECG machine that records signals in empirically determined locations on a human body, our approach is focused on recording three projections of the heart vector. The successful recording of the projections of the heart vector enables the synthesis of a 12-lead signal set and internal algorithmic diagnostic work in the space of 3D heart vectors.
There are obvious ease of use advantages when comparing our handheld device that fits in a wallet and can be instantly self-applied versus the current 12-lead ECG machine that requires a trained professional to apply. In addition, there are diagnostic performance advantages, including, based on our initial study, increased accuracy in diagnosing MIs. The system is used by patients at home or elsewhere, with help from their physicians, to assess whether their chest pain is truly the result of an MI.
Our telehealth HeartBeam AIMIGo system will be a prescription-only mobile health system intended for individuals with known or suspected heart disease, especially CAD. It helps guide physicians in choosing the best course of action for their patients who experience chest pain outside of a medical facility. HeartBeam AIMIGo will bring a medical grade ECG to patients and will enable them to receive a plan of action from a physician in a timely manner. At the time of onboarding and at regularly scheduled time intervals, patients record a baseline 30 second cardiac reading using our device. When a patient experiences symptoms, such as irregular heartbeats or chest pain, the patient can simply open the smartphone app and press the credit card sized device against the chest to collect signals that can be converted to a synthesized 12-lead ECG. This synthesized 12-lead ECG is sent to the physician overlayed with the patient’s derived baseline ECG recording. In addition, the patient provides input on their symptoms that are sent, along with the ECG data, to the cloud for interpretation by a physician. A cloud-based algorithm processes the signals and displays the symptom description and patient history to a physician to analyze and prescribe an action plan. From start to finish, the process takes just a few minutes.
The telehealth HeartBeam AIMIGo system consists of:
1. A credit card sized cardiac electrical signal collection device. The device captures cardiac signals that represent x, y and z projections of the heart vector and transmits them via Bluetooth connection to a smartphone. It is always with the patient as it easily fits in a wallet. It is easy to use as all that is required of the patient is that the device be pressed against the chest.
2. A smartphone application that receives the cardiac signals from the HeartBeam signal collection device. The app has several functions: guiding the patient through the signal collection, asking about symptoms, displaying the status of the data collection, and notifying the patient of the plan of action as determined by a physician. In addition, the app will contain HIPAA-compliant video conferencing or text capabilities for the healthcare provider to communicate directly with the patient.
3. A cloud-based software system that serves four basic functions: (1) Performing a final check of the ECG signal quality, (2) Synthesizing a 12-lead ECG from the measured (recorded) 3 vector leads, (3) A diagnostic suggestion based on 3D VECG interpretation, risk factors and symptoms and (4) Preparing a summary report for the physician. These software functions will be introduced to the HeartBeam AIMIGo product in a sequential manner. In order to facilitate a more accurate physician interpretation of the data, the software overlays the patient’s synthesized baseline 12 lead ECG waveform on the synthesized 12 lead ECG waveform from the current event. To ensure high signal quality, the system checks for noise levels in the recorded signals. Those signals that can be effectively filtered are accepted and those that have a noise level above an empirically established threshold are rejected. If a recorded signal is rejected, the user is asked to repeat the recording.
4
4. A web-based physician portal capable of displaying the relevant information for the physician to analyze: diagnostic suggestion, patient history, symptoms, baseline and current readings, synthesized 12-lead ECG, and recorded 3 vector leads. Our physician portal assists physicians with their diagnostic interpretation by providing both the baseline 12-lead synthesized ECG and the 12-lead synthesized ECG that is under evaluation.
The market release of our telehealth Product will be in multiple generations.
The generation 1 Product will have a limited feature set and introduce a 3-lead 3D VECG credit card-sized device, the HeartBeam AIMIGo 3L, that records the X,Y,Z cardiac activity and displays the signals for clinician review, providing the regulatory foundation for subsequent products in our product portfolio. The 510(K) submission to the FDA is planned for the fourth quarter of 2022.
The generation 2 Product will offer to the physician a pair of baseline and symptomatic 12-lead ECGs both synthesized from 3-lead 3D VECG signals recorded by the HeartBeam AIMIGo device and a symptoms report. It leverages recently issued patents for enhanced diagnostic accuracy of the synthesized 12-lead ECG waveforms. The generation 2 Product is planned to offer an automated atrial fibrillation detection algorithm. This product is an excellent match for existing CPT RPM reimbursement codes. The 510(K) submission to the FDA is planned for the second quarter of 2023.
The generation 3 Product will feature our proprietary MI marker as well as our diagnostic suggestion in addition to all features of the earlier generation Products.
We plan to seek a unique reimbursement code for HeartBeam AIMIGo.
The same core technology is used in the ED Product (HeartBeam AIMI). In this application, the increased accuracy of detecting MIs by the ECG is of utmost importance. An ECG is the first diagnostic test a chest pain patient receives in the ED and it has a major impact on the patient’s subsequent clinical path. The ED Product has no hardware and introduces minimal change to the standard of care chest pain diagnostic path. It uses a baseline standard 12-lead ECG from the patient’s Electronic Medical Record (“EMR”) and the chest pain ECG that is being evaluated. It converts them to a VECG representation and utilizes our proprietary 3D VECG differential marker. An initial clinical study indicates that the ED software Product offers considerable improvement in the accuracy of MI detection compared to a panel of experienced cardiologists interpreting a standard 12 lead ECG. The importance of increased accuracy of the first ECG interpretation for a chest pain patient presenting to an ED is significant, potentially leading to faster intervention or avoiding unnecessary activation of the cardiac catheterization lab.
Market Opportunity
ECGs are key diagnostic tests utilized in the diagnosis and monitoring of cardiovascular disease, the number one cause of death worldwide. In the US in 2016, there were 121.5 million adults living with cardiovascular disease and 18.3 million adults with diagnosed coronary artery disease. The market size is increasing, due to an aging population and lifestyle choices.
Every 40 seconds someone in the US has a heart attack (“MI”). Unfortunately, there is no way for patients to tell whether the symptoms they are experiencing are due to an MI, or some other more benign condition such as indigestion. As a result, patients often ignore symptoms and delay seeking care, which leads to worse outcomes and increased mortality. On the other hand, many patients who go to the Emergency Room with chest pain are not experiencing an MI. Chest pain is the second most common reason for an ED visit, yet fewer than 20% of chest pain ED visits result in a diagnosis of a life-threatening condition. These unnecessary ED visits lead to well over $10 billion in unnecessary healthcare expenditures.
Most ECGs are conducted in a healthcare facility setting using a 12-lead ECG machine, the gold standard. ECGs taken outside of healthcare facilities are expected to grow more quickly than in-hospital ECGs. Monitoring cardiac patients outside of a hospital is a fast-growing trend, as it is less expensive and provides a better patient experience. However, while ambulatory cardiac monitoring devices are often much easier for patients to use, they have fewer leads than the gold standard and therefore cannot offer as comprehensive a picture of cardiac health.
5
While a standard 12-lead ECG readout is of great medical value, it is simply impractical to have a machine next to patients when they experience symptoms outside the clinical setting, since recording the event requires attaching multiple electrodes to the patient’s body with professional assistance. While existing technologies use predominantly single lead ECG devices to monitor arrhythmias, these technologies do not provide information to the physician on the presence of life-threatening conditions of ACS or heart attacks.
We believe our telehealth technology addresses these market needs and has several key attributes that make it a good fit for these patients. Our telehealth Product is generally used when symptoms occur and offers the potential for lifelong patient usage. The device is always near the patient and ready to be used for recording a cardiac event. It enables real-time cardiac data transmission during a telemedicine visit. It offers a recorded 3 vector lead set of signals and a synthesized 12-lead ECG set of signals. We believe physicians will typically prescribe our solution to chronic cardiovascular patients for long term monitoring, thereby enabling prolonged data collection and delivering a more complete picture for diagnosis. This will also enable the use of artificial intelligence on our future database that will have a unique set of longitudinal synthesized 12-lead ECGs for patients.
Market Strategy
Our goal is to establish our products as key solutions for cardiology practices and hospital EDs. Our efforts to enter the market involve establishing clinical evidence and demonstrating the cost-effectiveness of adopting our products. For both the telehealth (HeartBeam AIMIGo) and the ED Products (HeartBeam AIMI), the initial geographic market is the United States.
We believe that both the telehealth and ED Products will be subject to the US FDA’s 510(k) review process. A 510(K) for our HeartBeam AIMI was submitted to the FDA on August 15, 2022 for review and we are in the process of preparing a 510(K) submission for HeartBeam AIMIGo.
For HeartBeam AIMIGo, the primary customers are cardiology practices and the cardiology departments of hospitals. Healthcare insurers are another important customer, as they will potentially benefit from the reduced costs to the healthcare system. We are working to develop new clinical studies and publish results of completed clinical studies and plan to demonstrate real world cost-effectiveness of the use of the solution.
Our long-term strategy is to generate sufficient evidence of clinical efficacy and cost-effectiveness to generate reimbursement coverage and payment specifically for the HeartBeam telehealth Generation 2 solution. We expect to be able to demonstrate significant clinical benefits for patients and savings to the health care system, justifying reimbursement levels well in excess of the amount paid through the RPM pathway.
For the telehealth Product, our primary marketing strategy will focus on the medical community with continued validation of clinical efficacy and cost-effectiveness and the establishment of reference sites. We will also create educational materials and provide other support to help educate our customers’ patients.
Clinical Data
HeartBeam has performed three clinical studies to assess performance of our technologies.
In the first study (HeartBeam Ischemia Detection Study — HIDES), we collected electrical signal data on patients whose coronary arteries were occluded during a Percutaneous Coronary Intervention (PCI), using simultaneously a traditional 12-lead ECG and our vector signal based device. Our VECG-based signal interpretation system had significantly (21%) higher accuracy in detecting ischemia as more fully discussed in the Business Section.
In a second study (B Score), the HeartBeam diagnostic engine, using ECG, symptoms, and history, matched the diagnostic performance of expert cardiologists in detecting the presence of MIs in patients presenting to an ED with chest pain. This result indicates that the quality of the diagnostic advice produced by our expert system will be extremely valuable to the physician who is assessing the condition of a patient in a telehealth environment.
The third study, ISPEC, which assessed the false positive rate for non-symptomatic patients, is relevant in a telehealth situation. It is important that the system have a low false positive rate when patients are conducting baseline recordings, which are required on at least a monthly basis. The study yielded no false positives.
All three studies are being prepared for peer-reviewed publication.
6
Intellectual Property
We believe our innovations are protected with our patent portfolio and our goal is to become a leader in the ambulatory VECG sector. For a limited number of aspects of our proprietary technology we rely on trade secret protection. It is our view that the combination of these two methods of intellectual property protection maximizes our chances for success.
The issued and pending U.S. patent applications cover compact VECG systems for remote detection and/or diagnosis of AMI. The pending EU and CN patent applications correspond to the pending and issued US cases. The pending PCT applications cover methods and apparatuses for automatic cardiac diagnosis as well as compact systems including retractable electrodes.
HeartBeam has five issued U.S. patents (U.S.10,433,744, U.S.10,117,592, U.S.11,071,490, U.S.11,419,538 and U.S. 11,445,963), and seven pending U.S applications. Five of the pending applications have been published, the remaining two pending cases are unpublished. Outside of the U.S., HeartBeam has four issued patents in Germany, France, Netherlands and United Kingdom and seven pending applications in Canada, China, the European Union (“EU”), Japan and Australia. HeartBeam has two pending PCT applications. The issued patents are predicted to expire between April 11, 2036 and October 5, 2041.
Research and Development
The primary objective of our research and development program is to provide innovative ambulatory VECG, user friendly solutions with high medical value. To date, we have been highly successful in developing our initial products. The emphasis has been on developing a user-friendly solution that is always with the patient and that provides assistance to physicians in diagnosing heart attacks in chest pain patients.
Our Research team is largely based in Belgrade, Serbia as well as in California, USA. We have assembled a highly capable Belgrade team currently consisting of seven PhD level contributors who are credited with developing our key inventions and patents. The diverse group includes:
• Two nuclear physicists.
• Two Biomedical Engineers experienced in developed digital health applications.
• Two highly experienced Healthcare IT development professionals.
• Two Electrical Engineers (M.S.E.E) with strong signal processing and ECG analysis algorithm expertise from the medical device industry.
• An Electrical Engineer (M.S.E.E) with exceptional implantable medical device development and power optimization expertise.
• A Software Engineer, (PhD Computer Science), with deep expertise in developing mobile applications for medical devices.
Future research and development efforts will focus on the application of signal processing and artificial intelligence to address a range of cardiac conditions.
In the third quarter of 2022 we added 3 development engineers led by our recently hired Chief Technology Officer Ken Persen. This is an experienced development team that worked together previously and was successful in delivering FDA cleared products.
Future Products
Our core technology — the heart vector approach adopted and invented by our research team — is a platform technology that can provide diagnostic solutions to a variety of cardiovascular patients. Our plans call for expanding solutions that diagnose all major cardiac conditions that are diagnosed by ECGs.
7
Our future plans include the development of a VECG that is a synthesized 12-lead capable patch ECG monitor that will provide advantages over existing single-lead ECG patch products such as the Zio-XT from iRhythm Technologies, Inc. Our approach will offer a synthesized 12-lead ECG with a patch that is very similar, in dimensions and look and feel, to the currently available single lead ECG patches. We believe providing standard of care 12-lead ECG capabilities will have significant diagnostic advantages over a single lead patch.
We also plan to integrate a synthesized 12-lead ECG smartwatch-based monitor intended for detection of heart attacks and complex cardiac arrhythmias. This invention eliminates the need for a dedicated ECG device while offering a synthesized 12-lead ECG capability enabling heart attack and complex arrhythmia detection.
While our initial telehealth Product is powered by a software expert system that serves as a diagnostic aid to a physician, we will develop an AI based diagnostic system that will supplement our diagnostic expert system.
We are committed to continue advancing the full potential inherent in our synthesized 12-lead 3D VECG technology as demonstrated in recently issued and allowed patents with potentially enormous market impacts.
Corporate Information
Our corporate offices are located at 2118 Walsh Avenue, Suite 210, Santa Clara, CA 95050. Our telephone number is (408) 899-4443. The address of our website is https://www.heartbeam/. The inclusion of our website address in this prospectus does not include or incorporate by reference the information on our website into this prospectus.
8
Investment in any securities offered pursuant to this prospectus and the applicable prospectus supplement involves risks. You should carefully consider the risk factors incorporated by reference to our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q or Current Reports on Form 8-K we file after the date of this prospectus, and all other information contained or incorporated by reference into this prospectus, as updated by our subsequent filings under the Exchange Act, and the risk factors and other information contained in the applicable prospectus supplement before acquiring any of such securities. The occurrence of any of these risks might cause you to lose all or part of your investment in the offered securities.
9
SPECIAL NOTICE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve risks and uncertainties, principally in the sections entitled “Risk Factors.” All statements other than statements of historical fact contained in this prospectus, including statements regarding future events, our future financial performance, business strategy and plans and objectives of management for future operations, are forward-looking statements. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” or “will” or the negative of these terms or other comparable terminology. Although we do not make forward looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks outlined under “Risk Factors” or elsewhere in this prospectus, which may cause our or our industry’s actual results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Forward-looking statements should not be read as a guarantee of future performance or results, and will not necessarily be accurate indications of the times at, or by which, that performance or those results will be achieved. Forward-looking statements are based on information available at the time they are made and/or management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from what is expressed in or suggested by the forward-looking statements.
Forward-looking statements speak only as of the date they are made. You should not put undue reliance on any forward-looking statements. We assume no obligation to update forward-looking statements to reflect actual results, changes in assumptions or changes in other factors affecting forward-looking information, except to the extent required by applicable securities laws. If we do update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
10
We intend to use the net proceeds from the sale of the securities as set forth in the applicable prospectus supplement.
11
The following description of the Company’s capital stock and provisions of its Certificate of Incorporation and Bylaws are summaries and are qualified by reference to the Company’s Certificate of Incorporation and Bylaws, which have been publicly filed with the SEC. See “Where You Can Find More Information” and “Incorporation by Reference.”
Authorized and Outstanding Capital Stock
The Company is authorized to issue 110,000,000 shares of capital stock, consisting of 100,000,000 shares of Common Stock, par value $0.0001 per share and 10,000,000 shares of Preferred Stock, par value $0.0001 per share.
As of December 31, 2022, the Company had 8,009,743 outstanding shares of Common Stock held by approximately 50 shareholders of record. As of the date thereof, there were no shares of preferred stock issued and outstanding.
Common Stock
The holders of our Common Stock are entitled to one vote per share. In addition, the holders of our Common Stock will be entitled to receive dividends ratably, if any, declared by our board of directors out of legally available funds; however, the current policy of the board of directors is to retain earnings, if any, for operations and growth. Upon liquidation, dissolution or winding-up, the holders of our Common Stock are entitled to share ratably in all assets that are legally available for distribution. The holders of our Common Stock have no pre-emptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of the our Common Stock are subject to, and may be adversely affected by, the rights of the holders of any series of preferred stock, which may be designated solely by action of the board of directors and issued in the future.
Preferred Stock
Our Board of Directors will have the authority, without further action by our stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of Common Stock. The issuance of our preferred stock could adversely affect the voting power of holders of Common Stock and the likelihood that such holders will receive dividend payments and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change in control of our company or other corporate action.
Warrants
The following summary of certain terms and provisions of the warrants (the “Warrants”) is not complete and is subject to, and qualified in its entirety by, the provisions of the warrant agency agreement between us and VStock Transfer, LLC (the “Warrant Agent”), and the form of warrant, which have been publicly filed with the SEC. See “Where You Can Find More Information” and “Incorporation by Reference.” As of December 31, 2022, there were 3,162,500 Warrants issued and outstanding trading under BEATW. The exercise price of the Warrants is $6 per share. Each Warrant is exercisable for one share of our Common Stock, subject to adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock as described herein. A holder may not exercise any portion of a warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% of the outstanding Common Stock after exercise, as such percentage ownership is determined in accordance with the terms of the Warrants, except that upon notice from the holder to us, the holder may waive such limitation up to a percentage, not in excess of 9.99%. Each warrant will be exercisable immediately upon issuance and will expire five (5) years after the initial issuance date.
12
In addition to the warrants mentioned above, the Company has issued the following:
• On February 18, 2022, the Company issued 58,000 warrants to purchase 58,000 shares of common stock at an exercise price of $6.00 per share, with an expiration date of five years from the date thereof.
• On January 14, 2022, the Company issued 72,727 warrants based on performance metrics achieved in 2021 to purchase 72,727 shares of common stock at an exercise price of $5.50 per share, with an expiration of five years from the date of issuance.
• In connection with the short-term notes issued in 2019, the Board of Directors approved the issuance of 15,277 fully vested warrants as an incentive to investors with the rights to convert into a fixed number of shares of the Company’s common stock for an above market fixed price of $2.75 per share, exercisable, in whole or in part, for a period of 4 years from the date of issuance.
• During the course of 2019, the Company issued milestone warrants totaling 407,272 units (“Penny Warrants”). These were valued with an exercise price of $0.0003 and will vest upon meeting certain milestones. The warrant may be exercised, in whole or in part upon the earliest to occur of: (i) following the Company’s initial public offering, the date on which the Company has a market capitalization of at least $50,000,000 for five consecutive business days; (ii) the closing of a Change of Control transaction with net proceeds to Company equity holders of at least $50,000,000; (iii) the date on which the Company receives a bona fide pre-money valuation from a third party investor of at least $50,000,000; (iv) the date on which the Holder’s continuous status as a Service Provider is terminated by the Company without Cause upon or within 12 months after a Change of Control; and (v) the date on which the Holder terminates his continuous status as a Service Provider for Good Reason within 12 months after a Change of Control.
• On November 15, 2022, the Company issued warrants to the lead underwriter, as portion of the underwriting compensation payable in connection with the Company’s initial public offering (“IPO”). The warrants accounted for 7% of the number of common stock sold in the IPO, which was 192,50 warrants, exercisable at a per share exercise price equal to $7.50 per share and expire five years from the date of issuance. The warrants are subject to a 180-day lock-up period.
2022 Equity Incentive Plan
On June 15, 2022, the our Board of Directors approved the 2022 Equity Incentive Plan (“2022 Equity Plan”), to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to employees, directors, and consultants, and to promote the success of the Company’s business. The 2022 Equity Plan provides for the grant of stock options and restricted stock awards (“RSUs”) to purchase common stock. The Board of Directors approved 1,900,000 shares of Common Stock issuance under the 2022 Equity Plan.
As of December 31, 2022, there were 747,364 shares available for issuance under the 2022 Equity Plan. The number of shares available for issuance under the 2022 Equity Plan will be increased on the first day of each fiscal year beginning with the 2023 fiscal year, in an amount equal to the least of 3,800,000 Shares, five percent (5%) of the total number of shares of all classes of common stock of the Company outstanding on the last day of the immediately preceding fiscal year, and a lesser number of Shares determined by the Administrator.
Eligible recipients of option awards are employees, officers, consultants, attorneys, advisors or directors (including non-employee directors) of the Company or of any parent, subsidiary or affiliate of the Company. The Board of Directors has the authority to grant to any eligible recipient any options, restricted stock or other awards valued in whole or in part by reference to, or otherwise based on, the Company’s Common Stock; provided, however, that Incentive Options may only be granted to employees of the Company or its subsidiaries.
The provisions of each option granted need not be the same with respect to each option recipient. Option recipients have entered into award agreements with the Company, in such form as the full Board of Directors has determined. The 2022 Equity Plan is administered by the Board of Directors.
13
Anti-Takeover Provisions
The following is a summary of certain provisions of Delaware law, our Certificate of Incorporation and our bylaws. This summary does not purport to be complete and is qualified in its entirety by reference to the corporate law of Delaware and our Certificate of Incorporation and bylaws.
Amended Certificate of Incorporation and Amended and Restated Bylaws
Our charter documents include provisions that may have the effect of discouraging, delaying or preventing a change in control or an unsolicited acquisition proposal that a stockholder might consider favorable, including a proposal that might result in the payment of a premium over the market price for the shares held by our stockholders. Certain provisions are summarized in the following paragraphs.
Effects of authorized but unissued common stock. One of the effects of the existence of authorized but unissued common stock may be to enable our board of directors to make more difficult or to discourage an attempt to obtain control of our Company by means of a merger, tender offer, proxy contest or otherwise, and thereby to protect the continuity of management. If, in the due exercise of its fiduciary obligations, the board of directors were to determine that a takeover proposal was not in our best interest, such shares could be issued by the board of directors without stockholder approval in one or more transactions that might prevent or render more difficult or costly the completion of the takeover transaction by diluting the voting or other rights of the proposed acquirer or insurgent stockholder group, by putting a substantial voting block in institutional or other hands that might undertake to support the position of the incumbent board of directors, by effecting an acquisition that might complicate or preclude the takeover, or otherwise.
Special Meeting of Stockholders and Stockholder Action by Written Consent. A special meeting of the stockholders may be called at any time by the Board, Chairperson of the Board, Chief Executive Officer or President (in the absence of a Chief Executive Officer) or by one or more stockholders holding shares in the aggregate entitled to cast not less than 10% of the votes at that meeting.
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the DGCL, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
• before such date, our board of directors approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder;
• upon closing of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned by (i) persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or on or after such date, the business combination is approved by our board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66-⅔% of the outstanding voting stock that is not owned by the interested stockholder.
In general, Section 203 defines business combination to include the following:
• any merger or consolidation involving the corporation and the interested stockholder;
• any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder;
• subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder;
14
• any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or
• The receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through the corporation.
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
The NASDAQ Capital Market Listing
Our common stock and warrants are listed on the NASDAQ, under the symbol “BEAT” and “BEATW”, respectively.
Transfer Agent and Warrant Agent
The transfer agent, warrant agent and registrar for our common stock is VStock Transfer with an address of 18 Lafayette Place Woodmere, New York 11598.
15
DESCRIPTION OF DEBT SECURITIES
General
The debt securities that we may offer by this prospectus consist of notes, debentures, or other evidences of indebtedness. The debt securities may constitute either senior or subordinated debt securities, and in either case may be either secured or unsecured. Any debt securities that we offer and sell will be our direct obligations. Debt securities may be issued in one or more series. All debt securities of any one series need not be issued at the same time, and unless otherwise provided, a series of debt securities may be reopened, with the required consent of the holders of outstanding debt securities, for issuance of additional debt securities of that series or to establish additional terms of that series of debt securities (with such additional terms applicable only to unissued or additional debt securities of that series). The form of indenture has been filed as an exhibit to the registration statement of which this prospectus is a part and is subject to any amendments or supplements that we may enter into with the trustee(s), however, we may issue debt securities not subject to the indenture provided such terms of debt securities are not otherwise required to be set forth in the indenture. The material terms of the indenture are summarized below and we refer you to the indenture for a detailed description of these material terms. Additional or different provisions that are applicable to a particular series of debt securities will, if material, be described in a prospectus supplement relating to the offering of debt securities of that series. These provisions may include, among other things and to the extent applicable, the following:
• the title of the debt securities, including, as applicable, whether the debt securities will be issued as senior debt securities, senior subordinated debt securities or subordinated debt securities, any subordination provisions particular to the series of debt securities;
• any limit on the aggregate principal amount of the debt securities;
• whether the debt securities are senior debt securities or subordinated debt securities and applicable subordination provisions, if any;
• whether the debt securities will be secured or unsecured;
• if other than 100% of the aggregate principal amount, the percentage of the aggregate principal amount at which we will sell the debt securities, such as an original issuance discount;
• the date or dates, whether fixed or extendable, on which the principal of the debt securities will be payable;
• the rate or rates, which may be fixed or variable, at which the debt securities will bear interest, if any, the date or dates from which any such interest will accrue, the interest payment dates on which we will pay any such interest, the basis upon which interest will be calculated if other than that of a 360-day year consisting of twelve 30-day months, and, in the case of registered securities, the record dates for the determination of holders to whom interest is payable;
• the place or places where the principal of and any premium or interest on the debt securities will be payable and where the debt securities may be surrendered for conversion or exchange;
• whether we may, at our option, redeem the debt securities, and if so, the price or prices at which, the period or periods within which, and the terms and conditions upon which, we may redeem the debt securities, in whole or in part, pursuant to any sinking fund or otherwise;
• if other than 100% of the aggregate principal amount thereof, the portion of the principal amount of the debt securities which will be payable upon declaration of acceleration of the maturity date thereof or provable in bankruptcy, or, if applicable, which is convertible or exchangeable;
• any obligation we may have to redeem, purchase or repay the debt securities pursuant to any sinking fund or analogous provisions or at the option of a holder of debt securities, and the price or prices at which, the currency in which and the period or periods within which, and the terms and conditions upon which, the debt securities will be redeemed, purchased or repaid, in whole or in part, pursuant to any such obligation, and any provision for the remarketing of the debt securities;
16
• the issuance of debt securities as registered securities or unregistered securities or both, and the rights of the holders of the debt securities to exchange unregistered securities for registered securities, or vice versa, and the circumstances under which any such exchanges, if permitted, may be made;
• the denominations, which may be in United States Dollars or in any foreign currency, in which the debt securities will be issued, if other than denominations of $1,000 and any integral multiple thereof;
• whether the debt securities will be issued in the form of certificated debt securities, and if so, the form of the debt securities (or forms thereof if unregistered and registered securities are issuable in that series), including the legends required by law or as we deem necessary or appropriate, the form of any coupons or temporary global security which may be issued and the forms of any other certificates which may be required under the indenture or which we may require in connection with the offering, sale, delivery or exchange of the debt securities;
• if other than United States Dollars, the currency or currencies in which payments of principal, interest and other amounts payable with respect to the debt securities will be denominated, payable, redeemable or repurchasable, as the case may be;
• whether the debt securities may be issuable in tranches;
• the obligations, if any, we may have to permit the conversion or exchange of the debt securities into common stock, preferred stock or other capital stock or property, or a combination thereof, and the terms and conditions upon which such conversion or exchange will be effected (including conversion price or exchange ratio), and any limitations on the ownership or transferability of the securities or property into which the debt securities may be converted or exchanged;
• if other than the trustee under the indenture, any trustees, authenticating or paying agents, transfer agents or registrars or any other agents with respect to the debt securities;
• any deletions from, modifications of or additions to the events of default with respect to the debt securities or the right of the Trustee or the holders of the debt securities in connection with events of default;
• any deletions from, modifications of or additions to the covenants with respect to the debt securities;
• if the amount of payments of principal of, and make-whole amount, if any, and interest on the debt securities may be determined with reference to an index, the manner in which such amount will be determined;
• whether the debt securities will be issued in whole or in part in the global form of one or more debt securities and, if so, the depositary for such debt securities, the circumstances under which any such debt security may be exchanged for debt securities registered in the name of, and under which any transfer of debt securities may be registered in the name of, any person other than such depositary or its nominee, and any other provisions regarding such debt securities;
• whether, under what circumstances and the currency in which, we will pay additional amounts on the debt securities to any holder of the debt securities who is not a United States person in respect of any tax, assessment or governmental charge and, if so, whether we will have the option to redeem such debt securities rather than pay such additional amounts, and the terms of any such option;
• whether the debt securities will be secured by any collateral and, if so, a general description of the collateral and the terms of any related security, pledge or other agreements;
• the persons to whom any interest on the debt securities will be payable, if other than the registered holders thereof on the regular record date therefore; and
• any other material terms or conditions upon which the debt securities will be issued.
Unless otherwise indicated in the applicable prospectus supplement, we will issue debt securities in fully registered form without coupons and in denominations of $1,000 and in integral multiples of $1,000, and interest will be computed on the basis of a 360-day year of twelve 30-day months. If any interest payment date or the maturity date falls on a day that is not a business day, then the payment will be made on the next business day without additional interest and with
17
the same effect as if it were made on the originally scheduled date. “Business day” means any calendar day that is not a Saturday, Sunday or legal holiday in New York, New York, and on which the trustee and commercial banks are open for business in New York, New York.
Unless we inform you otherwise in a prospectus supplement, each series of our senior debt securities will rank equally in right of payment with all of our other unsubordinated debt. The subordinated debt securities will rank junior in right of payment and be subordinate to all of our unsubordinated debt.
Unless otherwise indicated in the applicable prospectus supplement, the trustee will act as paying agent and registrar for the debt securities under the indenture. We may act as paying agent under the indenture.
The prospectus supplement will contain a description of United States federal income tax consequences relating to the debt securities, to the extent applicable.
Covenants
The applicable prospectus supplement will describe any covenants, such as restrictive covenants restricting us or our subsidiaries, if any, from incurring, issuing, assuming or guarantying any indebtedness or restricting us or our subsidiaries, if any, from paying dividends or acquiring any of our or its capital stock.
Consolidation, Merger and Transfer of Assets
The indenture permits a consolidation or merger between us and another entity and/or the sale, conveyance or lease by us of all or substantially all of our property and assets, provided that:
• the resulting or acquiring entity, if other than us, is organized and existing under the laws of a United States jurisdiction and assumes all of our responsibilities and liabilities under the indenture, including the payment of all amounts due on the debt securities and performance of the covenants in the indenture;
• immediately after the transaction, and giving effect to the transaction, no event of default under the indenture exists; and
• we have delivered to the trustee an officers’ certificate stating that the transaction and, if a supplemental indenture is required in connection with the transaction, the supplemental indenture comply with the indenture and that all conditions precedent to the transaction contained in the indenture have been satisfied.
If we consolidate or merge with or into any other entity, or sell or lease all or substantially all of our assets in compliance with the terms and conditions of the indenture, the resulting or acquiring entity will be substituted for us in the indenture and the debt securities with the same effect as if it had been an original party to the indenture and the debt securities. As a result, such successor entity may exercise our rights and powers under the indenture and the debt securities, in our name and, except in the case of a lease, we will be released from all our liabilities and obligations under the indenture and under the debt securities.
Notwithstanding the foregoing, we may transfer all of our property and assets to another entity if, immediately after giving effect to the transfer, such entity is our wholly owned subsidiary. The term “wholly owned subsidiary” means any subsidiary in which we and/or our other wholly owned subsidiaries, if any, own all of the outstanding capital stock.
Modification and Waiver
Under the indenture, some of our rights and obligations and some of the rights of the holders of the debt securities may be modified or amended with the consent of the holders of not less than a majority in aggregate principal amount of the outstanding debt securities affected by the modification or amendment. However, the following modifications and amendments will not be effective against any holder without its consent:
• a change in the stated maturity date of any payment of principal or interest;
• a reduction in the principal amount of or interest on any debt securities;
• an alteration or impairment of any right to convert at the rate or upon the terms provided in the indenture;
18
• a change in the currency in which any payment on the debt securities is payable;
• an impairment of a holder’s right to sue us for the enforcement of payments due on the debt securities; or
• a reduction in the percentage of outstanding debt securities required to consent to a modification or amendment of the indenture or required to consent to a waiver of compliance with certain provisions of the indenture or certain defaults under the indenture.
Under the indenture, the holders of not less than a majority in aggregate principal amount of the outstanding debt securities may, on behalf of all holders of the debt securities:
• waive compliance by us with certain restrictive provisions of the indenture; and
• waive any past default under the indenture in accordance with the applicable provisions of the indenture, except a default in the payment of the principal of or interest on any series of debt securities.
Events of Default
Unless we indicate otherwise in the applicable prospectus supplement, “event of default” under the indenture will mean, with respect to any series of debt securities, any of the following:
• failure to pay interest on any debt security for 30 days after the payment is due;
• failure to pay the principal of any debt security when due, either at maturity, upon redemption, by declaration or otherwise;
• failure on our part to observe or perform any other covenant or agreement in the indenture that applies to the debt securities for 90 days after we have received written notice of the failure to perform in the manner specified in the indenture; and
• certain events of bankruptcy, insolvency or reorganization.
Remedies Upon an Event of Default
If an event of default occurs and continues, the trustee or the holders of not less than 25% in aggregate principal amount of the outstanding debt securities of such series may declare the entire principal of all the debt securities to be due and payable immediately, except that, if the event of default is caused by certain events in bankruptcy, insolvency or reorganization, the entire principal of all of the debt securities of such series will become due and payable immediately without any act on the part of the trustee or holders of the debt securities. If such a declaration occurs, the holders of a majority of the aggregate principal amount of the outstanding debt securities of such series can, subject to conditions, rescind the declaration.
The indenture requires us to furnish to the trustee not less often than annually, a certificate from our principal executive officer, principal financial officer or principal accounting officer, as the case may be, as to such officer’s knowledge of our compliance with all conditions and covenants under the indenture. The trustee may withhold notice to the holders of debt securities of any default, except defaults in the payment of principal of or interest on any debt securities if the trustee in good faith determines that the withholding of notice is in the best interests of the holders. For purposes of this paragraph, “default” means any event which is, or after notice or lapse of time or both would become, an event of default under the indenture.
The trustee is not obligated to exercise any of its rights or powers under the indenture at the request, order or direction of any holders of debt securities, unless the holders offer the trustee satisfactory security or indemnity. If satisfactory security or indemnity is provided, then, subject to other rights of the trustee, the holders of a majority in aggregate principal amount of the outstanding debt securities may direct the time, method and place of:
• conducting any proceeding for any remedy available to the trustee; or
• exercising any trust or power conferred upon the trustee.
19
The holder of a debt security will have the right to begin any proceeding with respect to the indenture or for any remedy only if:
• the holder has previously given the trustee written notice of a continuing event of default;
• the holders of not less than a majority in aggregate principal amount of the outstanding debt securities have made a written request of, and offered reasonable indemnity to, the trustee to begin such proceeding;
• the trustee has not started such proceeding within 60 days after receiving the request; and
• no direction inconsistent with such written request has been given to the trustee under the indenture.
However, the holder of any debt security will have an absolute right to receive payment of principal of and interest on the debt security when due and to institute suit to enforce this payment.
Satisfaction and Discharge; Defeasance
Satisfaction and Discharge of Indenture. Unless otherwise indicated in the applicable prospectus supplement, if at any time,
• we have paid the principal of and interest on all the debt securities of any series, except for debt securities which have been destroyed, lost or stolen and which have been replaced or paid in accordance with the indenture, as and when the same shall have become due and payable, or
• we have delivered to the trustee for cancellation all debt securities of any series theretofore authenticated, except for debt securities of such series which have been destroyed, lost or stolen and which have been replaced or paid as provided in the indenture, or
• all the debt securities of such series not theretofore delivered to the trustee for cancellation have become due and payable, or are by their terms are to become due and payable within one year or are to be called for redemption within one year, and we have deposited with the trustee, in trust, sufficient money or government obligations, or a combination thereof, to pay the principal, any interest and any other sums due on the debt securities, on the dates the payments are due or become due under the indenture and the terms of the debt securities,
then the indenture shall cease to be of further effect with respect to the debt securities of such series, except for:
• rights of registration of transfer and exchange, and our right of optional redemption;
• substitution of mutilated, defaced, destroyed, lost or stolen debt securities;
• rights of holders to receive payments of principal thereof and interest thereon upon the original stated due dates therefor (but not upon acceleration) and remaining rights of the holders to receive mandatory sinking fund payments, if any;
• the rights, obligations and immunities of the trustee under the indenture; and
• the rights of the holders of such series of debt securities as beneficiaries thereof with respect to the property so deposited with the trustee payable to all or any of them.
Defeasance and Covenant Defeasance. Unless otherwise indicated in the applicable prospectus supplement, we may elect with respect to any debt securities of any series either:
• to defease and be discharged from all of our obligations with respect to such debt securities (“defeasance”), with certain exceptions described below; or
• to be released from our obligations with respect to such debt securities under such covenants as may be specified in the applicable prospectus supplement, and any omission to comply with those obligations will not constitute a default or an event of default with respect to such debt securities (“covenant defeasance”).
20
We must comply with the following conditions before the defeasance or covenant defeasance can be effected:
• we must irrevocably deposit with the indenture trustee or other qualifying trustee, under the terms of an irrevocable trust agreement in form and substance satisfactory to the trustee, trust funds in trust solely for the benefit of the holders of such debt securities, sufficient money or government obligations, or a combination thereof, to pay the principal, any interest and any other sums on the due dates for those payments; and
• we must deliver to the trustee an opinion of counsel to the effect that the holders of such debt securities will not recognize income, gain or loss for federal income tax purposes as a result of defeasance or covenant defeasance, as the case may be, to be effected with respect to such debt securities and will be subject to federal income tax on the same amount, in the same manner and at the same times as would be the case if such defeasance or covenant defeasance, as the case may be, had not occurred.
In connection with defeasance, any irrevocable trust agreement contemplated by the indenture must include, among other things, provision for:
• payment of the principal of and interest on such debt securities, if any, appertaining thereto when due (by redemption, sinking fund payments or otherwise),
• the payment of the expenses of the trustee incurred or to be incurred in connection with carrying out such trust provisions,
• rights of registration, transfer, substitution and exchange of such debt securities in accordance with the terms stated in the indenture, and
• continuation of the rights, obligations and immunities of the trustee as against the holders of such debt securities as stated in the indenture.
The accompanying prospectus supplement may further describe any provisions permitting or restricting defeasance or covenant defeasance with respect to the debt securities of a particular series.
Global Securities
Unless otherwise indicated in the applicable prospectus supplement, each debt security offered by this prospectus will be issued in the form of one or more global debt securities representing all or part of that series of debt securities. This means that we will not issue certificates for that series of debt securities to the holders. Instead, a global debt security representing that series will be deposited with, or on behalf of, a securities depositary and registered in the name of the depositary or a nominee of the depositary. Any such depositary must be a clearing agency registered under the Exchange Act. We will describe the specific terms of the depositary arrangement with respect to a series of debt securities to be represented by a global security in the applicable prospectus supplement.
Notices
We will give notices to holders of the debt securities by mail at the addresses listed in the security register. In the case of notice in respect of unregistered securities or coupon securities, we may give notice by publication in a newspaper of general circulation in New York, New York.
Governing Law
The particular terms of a series of debt securities will be described in a prospectus supplement relating to such series of debt securities. Any indentures will be subject to and governed by the Trust Indenture Act of 1939, as amended, and may be supplemented or amended from time to time following their execution. Unless otherwise stated in the applicable prospectus supplement, we will not be limited in the amount of debt securities that we may issue, and neither the senior debt securities nor the subordinated debt securities will be secured by any of our property or assets. Thus, by owning debt securities, you are one of our unsecured creditors.
Regarding the Trustee
From time to time, we may maintain deposit accounts and conduct other banking transactions with the trustee to be appointed under the indenture or its affiliates in the ordinary course of business.
21
We may offer to sell warrants from time to time. If we do so, we will describe the specific terms of the warrants in a prospectus supplement. In particular, we may issue warrants for the purchase of common stock, preferred stock and/or debt securities in one or more series. We may also issue warrants independently or together with other securities and the warrants may be attached to or separate from those securities.
We will evidence each series of warrants by warrant certificates that we will issue under a separate agreement. We will enter into the warrant agreement with a warrant agent. We will indicate the name and address of the warrant agent in the applicable prospectus supplement relating to a particular series of warrants.
We will describe in the applicable prospectus supplement the terms of the series of warrants, including:
• the offering price and aggregate number of warrants offered;
• the currency for which the warrants may be purchased;
• if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security;
• if applicable, the date on and after which the warrants and the related securities will be separately transferable;
• in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at, and currency in which, this principal amount of debt securities may be purchased upon such exercise;
• in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise;
• the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants;
• the terms of any rights to redeem or call the warrants;
• any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants;
• the dates on which the right to exercise the warrants will commence and expire;
• the manner in which the warrant agreement and warrants may be modified;
• certain United States federal income tax consequences of holding or exercising the warrants;
• the terms of the securities issuable upon exercise of the warrants; and
• any other specific material terms, preferences, rights or limitations of or restrictions on the warrants.
Holders may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with other requested information, and paying the required amount to the warrant agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth in the applicable prospectus supplement the information that the holder of the warrant will be required to deliver to the warrant agent.
Upon receipt of the required payment and the warrant certificate properly completed and duly executed at the office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise. If a holder exercises fewer than all of the warrants represented by the warrant certificate, then we will issue a new warrant certificate for the remaining amount of warrants.
22
Holder will not have any of the rights of the holders of the securities purchasable upon the exercise of warrants until you exercise them. Accordingly, holder will not be entitled to, among other things, vote or receive dividend payments or similar distributions on the securities you can purchase upon exercise of the warrants.
The information provided above is only a summary of the terms under which we may offer warrants for sale. Accordingly, investors must carefully review the applicable warrant agreement for more information about the specific terms and conditions of these warrants before investing in us. In addition, please carefully review the information provided in the applicable prospectus supplement, which contains additional information that is important for you to consider in evaluating an investment in our securities.
23
We may issue rights to our stockholders to purchase shares of our common stock or preferred stock described in this prospectus. We may offer rights separately or together with one or more additional rights, preferred stock, common stock, warrants or any combination of those securities in the form of units, as described in the applicable prospectus supplement. Each series of rights will be issued under a separate rights agreement to be entered into between us and a bank or trust company, as rights agent. The rights agent for any rights we offer will be set forth in the applicable prospectus supplement. The rights agent will act solely as our agent in connection with the certificates relating to the rights of the series of certificates and will not assume any obligation or relationship of agency or trust for or with any holders of rights certificates or beneficial owners of rights. The following description sets forth certain general terms and provisions of the rights to which any prospectus supplement may relate. The particular terms of the rights to which any prospectus supplement may relate and the extent, if any, to which the general provisions may apply to the rights so offered will be described in the applicable prospectus supplement. To the extent that any particular terms of the rights, rights agreement or rights certificates described in a prospectus supplement differ from any of the terms described below, then the terms described below will be deemed to have been superseded by that prospectus supplement. We encourage you to read the applicable rights agreement and rights certificate for additional information before you decide whether to purchase any of our rights.
The prospectus supplement relating to any rights that we offer will include specific terms relating to the offering, including, among other matters:
• the date of determining the stockholders entitled to the rights distribution;
• the aggregate number of shares of common stock, preferred stock or other securities purchasable upon exercise of the rights;
• the exercise price;
• the aggregate number of rights issued;
• whether the rights are transferrable and the date, if any, on and after which the rights may be separately transferred;
• the date on which the right to exercise the rights will commence, and the date on which the right to exercise the rights will expire;
• the method by which holders of rights will be entitled to exercise;
• the conditions to the completion of the offering;
• the withdrawal, termination and cancellation rights;
• whether there are any backstop or standby purchaser or purchasers and the terms of their commitment;
• whether stockholders are entitled to oversubscription right;
• any U.S. federal income tax considerations; and
• any other terms of the rights, including terms, procedures and limitations relating to the distribution, exchange and exercise of the rights.
If less than all of the rights issued in any rights offering are exercised, we may offer any unsubscribed securities directly to persons other than stockholders, to or through agents, underwriters or dealers or through a combination of such methods, including pursuant to standby arrangements, as described in the applicable prospectus supplement. In connection with any rights offering, we may enter into a standby underwriting or other arrangement with one or more underwriters or other persons pursuant to which such underwriters or other persons would purchase any offered securities remaining unsubscribed for after such rights offering.
24
We may issue units consisting of any combination of the other types of securities offered under this prospectus in one or more series. We may evidence each series of units by unit certificates that we will issue under a separate agreement. We may enter into unit agreements with a unit agent. We will indicate the name and address of the unit agent in the applicable prospectus supplement relating to a particular series of units.
The following description, together with the additional information included in any applicable prospectus supplement, summarizes the general features of the units that we may offer under this prospectus. You should read any prospectus supplement and any free writing prospectus that we may authorize to be provided to you related to the series of units being offered, as well as the complete unit agreements that contain the terms of the units. Specific unit agreements will contain additional important terms and provisions and we will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from another report that we file with the SEC, the form of each unit agreement relating to units offered under this prospectus.
If we offer any units, certain terms of that series of units will be described in the applicable prospectus supplement, including, without limitation, the following, as applicable:
• the title of the series of units;
• identification and description of the separate constituent securities comprising the units;
• the price or prices at which the units will be issued;
• the date, if any, on and after which the constituent securities comprising the units will be separately transferable;
• a discussion of certain United States federal income tax considerations applicable to the units; and
• any other terms of the units and their constituent securities.
25
We may sell our securities from time to time pursuant to underwritten public offerings, negotiated transactions, block trades or a combination of these methods or through underwriters or dealers, through agents and/or directly to one or more purchasers. Our securities may be distributed from time to time in one or more transactions:
• at a fixed price or prices, which may be changed;
• at market prices prevailing at the time of sale;
• at prices related to such prevailing market prices; or
• at negotiated prices.
Each time that we sell securities covered by this prospectus, we will provide a prospectus supplement or supplements that will describe the method of distribution and set forth the terms and conditions of the offering of such securities, including the offering price of the securities and the proceeds to us, if applicable.
Offers to purchase the securities being offered by this prospectus may be solicited directly. Agents may also be designated to solicit offers to purchase the securities from time to time. Any agent involved in the offer or sale of our securities will be identified in a prospectus supplement.
If a dealer is utilized in the sale of the securities being offered by this prospectus, the securities will be sold to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale.
If an underwriter is utilized in the sale of the securities being offered by this prospectus, an underwriting agreement will be executed with the underwriter at the time of sale and the name of any underwriter will be provided in the prospectus supplement that the underwriter will use to make resales of the securities to the public. In connection with the sale of the securities, we or the purchasers of securities for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting discounts or commissions. The underwriter may sell the securities to or through dealers, and those dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters and/or commissions from the purchasers for which they may act as agent. Unless otherwise indicated in a prospectus supplement, an agent will be acting on a best efforts basis and a dealer will purchase securities as a principal, and may then resell the securities at varying prices to be determined by the dealer.
Any compensation paid to underwriters, dealers or agents in connection with the offering of the securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers will be provided in the applicable prospectus supplement. Underwriters, dealers and agents participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act of 1933, as amended, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil liabilities, including liabilities under the Securities Act, or to contribute to payments they may be required to make in respect thereof and to reimburse those persons for certain expenses.
Any common stock will be listed on the Nasdaq Capital Market, but any other securities may or may not be listed on a national securities exchange. To facilitate the offering of securities, certain persons participating in the offering may engage in transactions that stabilize, maintain or otherwise affect the price of the securities. This may include over-allotments or short sales of the securities, which involve the sale by persons participating in the offering of more securities than were sold to them. In these circumstances, these persons would cover such over-allotments or short positions by making purchases in the open market or by exercising their over-allotment option, if any. In addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating in the offering may be reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. These transactions may be discontinued at any time.
We may engage in at the market offerings into an existing trading market in accordance with Rule 415(a)(4) under the Securities Act.
26
In addition, we may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement so indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and, if not identified in this prospectus, will be named in the applicable prospectus supplement (or a post-effective amendment). In addition, we may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities short using this prospectus and an applicable prospectus supplement. Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
We do not make any representation or prediction as to the direction or magnitude of any effect that the transactions described above might have on the price of the securities. In addition, we do not make any representation that underwriters will engage in such transactions or that such transactions, once commenced, will not be discontinued without notice.
The specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
To comply with applicable state securities laws, the securities offered by this prospectus will be sold, if necessary, in such jurisdictions only through registered or licensed brokers or dealers. In addition, securities may not be sold in some states unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
The underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business for which they receive compensation.
27
Lucosky Brookman LLP will pass upon certain legal matters relating to the issuance and sale of the securities offered hereby on behalf of HeartBeam, Inc. Additional legal matters may be passed upon for us or any underwriters, dealers or agents, by counsel that we will name in the applicable prospectus supplement.
The financial statements of HeartBeam, Inc. as of December 31, 2021 and 2020, and for each of the years in the two year period ended December 31, 2021, incorporated by reference in this Registration Statement, have been included in reliance upon the reports of Friedman LLP, an independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
The SEC maintains a web site that contains reports, proxy and information statements and other information about issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov.
Our website address is https://www.heartbeam.com. The information on our website, however, is not, and should not be deemed to be, a part of this prospectus.
This prospectus and any prospectus supplement are part of a registration statement that we filed with the SEC and do not contain all of the information in the registration statement. The full registration statement may be obtained from the SEC or us, as provided below. Forms of the documents establishing the terms of the offered securities are or may be filed as exhibits to the registration statement. Statements in this prospectus or any prospectus supplement about these documents are summaries and each statement is qualified in all respects by reference to the document to which it refers. You should refer to the actual documents for a more complete description of the relevant matters. You may inspect a copy of the registration statement through the SEC’s website, as provided above.
28
The SEC’s rules allow us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and subsequent information that we file with the SEC will automatically update and supersede that information. Any statement contained in a previously filed document incorporated by reference will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus modifies or replaces that statement.
We incorporate by reference our documents listed below and any future filings made by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, which we refer to as the “Exchange Act” in this prospectus, between the date of this prospectus and the termination of the offering of the securities described in this prospectus. We are not, however, incorporating by reference any documents or portions thereof, whether specifically listed below or filed in the future, that are not deemed “filed” with the SEC, including any information furnished pursuant to Items 2.02 or 7.01 of Form 8-K or related exhibits furnished pursuant to Item 9.01 of Form 8-K.
This prospectus and any accompanying prospectus supplement incorporate by reference the documents set forth below that have previously been filed with the SEC:
• Our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 24, 2022.
• Our Quarterly Report on Form 10-Q for the quarter ended March 31, 2022, June 30, 2022 and September 30, filed with the SEC on May 12, 2022, August 11, 2022 and November 10, 2022 respectively.
• Our Current Reports on Form 8-K filed with the SEC on February 2, 2022, February 22, 2022, March 10, 2022, June 16, 2022, August 8, 2022, August 12, 2022, September 21, 2022, November 17, 2022, January 24, 2023, March 3, 2023, and March 8, 2023.
• The information specifically incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2021 from our definitive proxy statement for the annual meeting of stockholders held on June 15, 2022, filed with the SEC on May 2, 2022.
• Our proxy statement for the special meeting of stockholders was held on November 14, 2022, filed with the SEC on October 11, 2022.
• The description of our securities contained in our Registration Statement on Form 8-A filed with the SEC on November 10, 2021, including any amendment or report filed for the purpose of updating such description.
All reports and other documents we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of this offering, including all such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated by reference into this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
You may request a free copy of any of the documents incorporated by reference in this prospectus (other than exhibits, unless they are specifically incorporated by reference in the documents) by writing or telephoning us at the following address:
Branislav Vajdic
Chief Executive Officer
2118 Walsh Avenue, Suite 210
Santa Clara, CA 95050
Telephone: 408-899-4443
Exhibits to the filings will not be sent, however, unless those exhibits have specifically been incorporated by reference in this prospectus and any accompanying prospectus supplement.
29
1,000,000
Shares of Common Stock
HeartBeam, Inc.
____________________________
Prospectus Supplement
____________________________
May 4, 2023